Palladium-Discovery, Properties, And Applications
Palladium is a chemical element that has the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal that belongs to the platinum group of elements. Palladium has a wide range of applications due to its unique properties, including its ability to absorb large amounts of hydrogen.
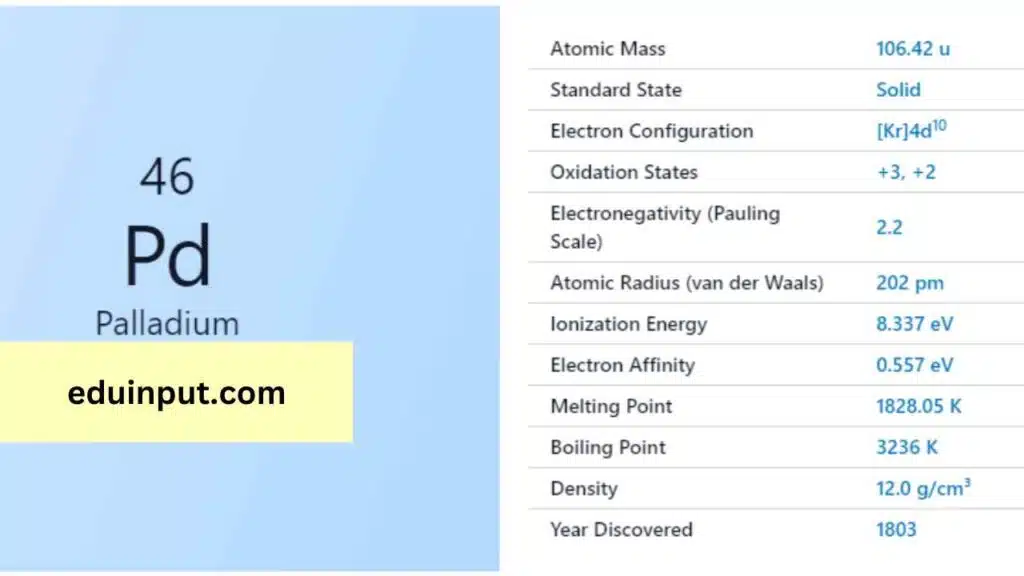
| Property | Value |
| Name | Palladium |
| Symbol | Pd |
| Atomic number | 46 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery white metallic |
| Classification | Metallic |
| The group in the periodic table | 10 |
| Group name | Precious metal or Platinum group metal |
| Period in periodic table | 5 |
| Period in the periodic table | d |
| Shell structure | 2.8.18.18.0 |
| CAS Registry | 7440-05-3 |
Discovery
Palladium was discovered in 1803 by William Hyde Wollaston, an English chemist, while he was studying the properties of platinum. Wollaston discovered palladium in the black residue left behind after dissolving platinum ore in aqua regia.
Physical Properties
Palladium is a soft, ductile, and malleable metal with a silvery-white appearance. It has a melting point of 1,554.9°C and a boiling point of 2,963°C. Palladium has a density of 12.02 g/cm³, which is similar to that of platinum. It is a good conductor of electricity and has a high resistance to corrosion and tarnishing.
Chemical Properties
Palladium is a highly reactive metal that readily reacts with oxygen, sulfur, and halogens. It has the ability to absorb up to 900 times its own volume of hydrogen, making it an important component in catalytic converters used in automobiles. Palladium also has catalytic properties in various chemical reactions, including the hydrogenation of unsaturated hydrocarbons.
Facts
- Palladium is one of the least dense metals in the platinum group.
- Palladium is used in dental fillings, jewelry, and watchmaking.
- Russia and South Africa are the largest producers of palladium in the world.
- Palladium is a byproduct of nickel, copper, and platinum mining.
Applications
Palladium has a wide range of applications due to its unique properties. It is commonly used in catalytic converters in automobiles to convert harmful pollutants into less harmful substances. Palladium is also used in electronics, such as capacitors and connectors, and in the production of LCD screens.
It is also used in dentistry, jewelry making, and watchmaking due to its resistance to tarnishing and corrosion. In addition, palladium is used in the chemical industry as a catalyst in various chemical reactions.







Leave a Reply