First Law Of Thermodynamics- Definition, and Examples
The first law of thermodynamics explains the relation between heat, work, and internal energy. The general principles that deal with heat energy and its transformation into mechanical energy are known as the laws of thermodynamics.
Discover real-world conduction examples that align with the first law of thermodynamics.
The first law of Thermodynamics
In any thermodynamic process, when heat Q is added to a system, energy appears as an increase in the internal energy ΔU stored in the system plus the work W done by the system on its surroundings. This is 1st law of thermodynamics is also called the law of conservation of energy.
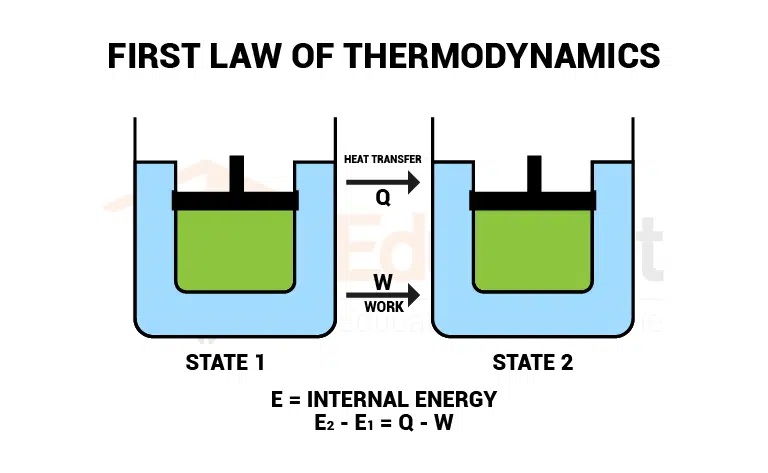
The first law of thermodynamics equation
The mathematically first law of thermodynamics is defined as
Q=ΔU+W
Consider some gas in a cylinder to which heat ‘Q’ is supplied. Due to the heat, the internal energy of the system changes from state U1 to state U2, and the system does some work ‘W’ on the surroundings.
Q= (U2-U1) +W
Q=ΔU+W
Which is the first law of thermodynamics
The first law of thermodynamics examples
There are many examples of the first law of thermodynamics
Bicycle Pump:
When we pump on the handle rapidly, it becomes hot. It is due to work done on the gas by which the internal energy of the gas increases. A bicycle pump with a blocked outlet is used To check the temperature of the air, a thermocouple is connected to the outlet. When the piston is pushed rapidly, the voltmeter shows deflection, which means that the air temperature has risen.
Human Metabolism:
Human beings and other animals do work when they walk, run or move heavy objects. Work requires energy. Energy is also needed for growth to make new cells and to replace old cells that have died.
These energy transforming processes are called Metabolism. From the first law of thermodynamic ΔU=Q-W
Due to work done, the internal energy decreases. So the body temperature or internal energy is maintained by the food we eat.
Types of Thermodynamics process
There are four types of thermodynamics process
- Isobaric process
- Isochoric process
- Isothermal process
- Adiabatic process
Isothermal process
A process in which the temperature of the system remains constant is called the isothermal process. So for an Isothermal process, Boyle’s law can be applied.
Adiabatic process
A process in which no heat enters or leaves the system is called an adiabatic process.
Isobaric process
An Isobaric process is a thermodynamic process in which the pressure of the system remains constant.
Isochoric process
An Isobaric process is a thermodynamic process in which the volume of the system remains constant.






Leave a Reply