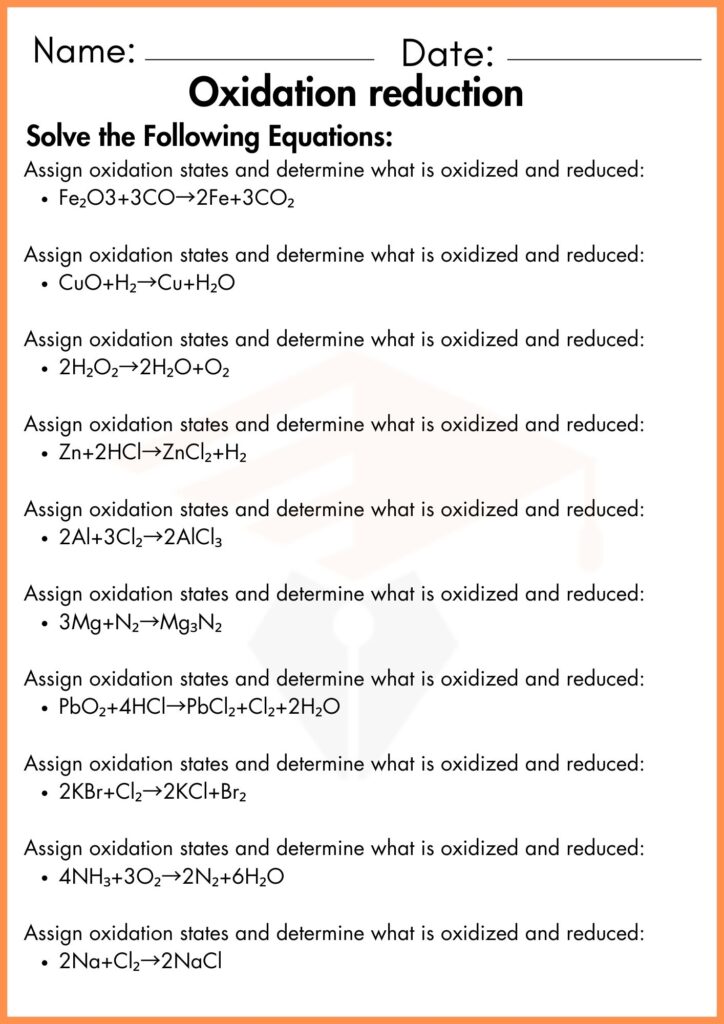
Oxidation Reduction Worksheets
The Oxidation-Reduction Worksheet is designed to help students understand and practice the concepts of oxidation and reduction reactions. In these reactions, one substance loses electrons (oxidation), and another gains electrons (reduction). The worksheet covers important topics like identifying the oxidizing and reducing agents, understanding oxidation numbers, and writing half-reactions. It also includes examples and problems that will guide students in solving these reactions step by step.
The Oxidation-Reduction Worksheet PDF is available for easy download and printing. With this, students can complete their exercises at their convenience. The Oxidation-Reduction Worksheet with answers PDF allows students to check their work and see how they can improve their problem-solving techniques. For more practice, the Oxidizing and Reducing Agents Practice Problems With Answers section helps students strengthen their knowledge of agents involved in these reactions. The Redox Half Reactions Worksheet with Answers and Oxidation Numbers Worksheet further break down the complex concepts into manageable tasks, allowing students to build their understanding progressively. If you need additional help, the Chapter 20 Worksheet: Redox Answer Key provides detailed solutions to assist students in learning more effectively.
Printable Oxidation Reduction Worksheets



Benefits of Using This Worksheet
The Oxidation-Reduction Worksheet is a great tool for students to improve their understanding of key chemistry concepts such as oxidation, reduction, and oxidation numbers. It provides hands-on practice that helps students apply theoretical knowledge to real-world problems. By working through the problems, students can develop a deeper understanding of topics like identifying oxidizing and reducing agents, writing half-reactions, and balancing equations.



Leave a Reply