Examples of Osmosis in Real Life and Biology
Osmosis is the movement of water across a semi-permeable membrane from an area of low solute concentration to high solute concentration. It’s a passive but essential process in biology, agriculture, and everyday life. From how plant roots absorb water to how kidneys concentrate urine and how pickles stay preserved, osmosis in real life influences hydration, nutrient absorption, and food storage. In this article, we will discuss 15 real-world examples of osmosis across plants, animals, humans, and medical applications.
Osmosis is driven by the difference in water potential between the two sides of the membrane.
Also, learn about Examples of Diffusion
Examples of Osmosis
Here are real-life examples of osmosis:
Osmosis in Plants and Agriculture
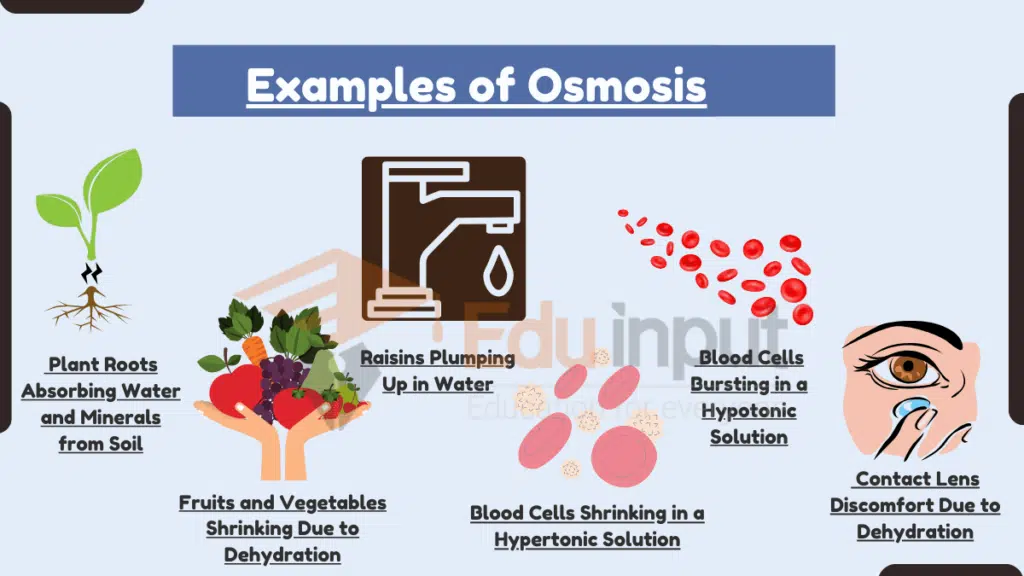
1. Water and Mineral Absorption by Roots
Plant roots absorb water and dissolved minerals from the surrounding soil primarily through osmosis. Soil typically has a higher water potential than the root cells, which contain many dissolved solutes like salts and sugars. This difference causes water to move into the root cells via osmosis, where it helps maintain cell turgor and transports nutrients upward through the plant’s vascular system.
For example, when soil is moist, water naturally enters root hair cells through their semipermeable membranes to balance internal solute concentrations, enabling the plant to grow and stay hydrated.
2. Shriveling of Fruits and Vegetables
When fruits and vegetables are left in dry environments, such as a refrigerator or countertop, they often shrink and wrinkle. This happens because the surrounding air has a lower water potential than the water-rich cells in the produce. Water diffuses from the produce into the dry air via osmosis, leading to dehydration and shrinkage.
Apples, for instance, lose moisture to the air, which causes their skin to wrinkle and the fruit to soften—a visible sign of osmosis at work in food storage.
3. Plumping of Raisins in Water
Raisins, being dried grapes, have a low water content and high sugar concentration. When placed in a glass of pure water, osmosis drives water from the high-potential environment (the glass) into the low-potential interior of the raisin. This rehydrates the raisin and causes it to swell and plump up.
This simple kitchen experiment perfectly illustrates osmotic movement driven by solute gradients.
4. Water Regulation via Stomata
Plants regulate water content through tiny openings on leaves called stomata. When the air inside a plant has a lower water potential than the surrounding environment, stomata open to allow water vapor to diffuse inward. Similarly, stomata help release excess water vapor through transpiration when needed, maintaining internal water balance.
The dynamic opening and closing of stomata allow plants to manage hydration and survive in varying environmental conditions—all governed by osmotic gradients.
Osmosis in Human and Animal Physiology
5. Shrinking of Blood Cells in Hypertonic Solutions
When red blood cells are placed in a hypertonic solution—one with a higher solute concentration than the fluid inside the cells—water exits the cells through osmosis. This loss of water causes the cells to shrink and develop a spiky appearance in a process called crenation.
This effect is critical in medical settings, such as administering intravenous fluids. Using the wrong concentration can lead to severe cellular dehydration.
6. Bursting of Blood Cells in Hypotonic Solutions
In contrast, placing red blood cells in a hypotonic solution—one with a lower solute concentration than the cell interior—causes water to flood into the cells. The influx of water through osmosis leads to swelling, and if unchecked, the cells can burst (hemolysis).
This is why hospitals use isotonic saline for IV fluids, maintaining balance and preventing osmotic damage to cells.
7. Thirst and Urination in Diabetes
Diabetes affects osmosis in the body due to elevated blood glucose levels. When blood sugar is high, it increases the solute concentration in the blood plasma. As a result, water is drawn from body cells into the bloodstream through osmosis. This dehydration triggers excessive thirst and increased urination.
Diabetics often experience dry mouth and frequent urination, classic symptoms that are driven by osmotic imbalance.
8. Dehydration in Cystic Fibrosis
Cystic fibrosis causes the body to produce thick mucus with a high electrolyte concentration. This mucus draws water from nearby epithelial cells through osmosis, leading to cellular dehydration and sticky secretions in the lungs and digestive tract.
The water loss worsens mucus buildup, making it harder for patients to breathe and increasing susceptibility to infections.
9. Urine Concentration by the Kidneys
Human kidneys rely on osmosis to filter blood and concentrate urine. Inside the nephrons—the kidney’s filtering units—water moves across membranes based on surrounding solute gradients. As the filtrate passes through different tubules, osmosis pulls water back into the bloodstream, concentrating the remaining waste.
This natural filtration process enables the body to retain water and eliminate harmful waste products.
10. Nutrient Absorption in Bacteria
Bacteria, too, rely on osmosis to absorb nutrients. When in environments with high-solute surroundings, bacteria take in water and dissolved substances through their semipermeable membranes to balance internal and external concentrations.
This mechanism allows bacteria to thrive in nutrient-rich or even extreme environments by adjusting their internal water content via osmosis.
Osmosis in Everyday and Medical Applications
11. Contact Lens Discomfort
Wearing dry contact lenses for extended periods often causes discomfort due to osmotic water loss. The dehydrated lenses have a higher solute concentration than the tear film on the eye. This causes water to move from the eye’s surface into the lens, leaving the eye feeling dry and irritated.
Using lens rewetting drops helps replenish moisture and restore osmotic balance.
12. Dialysis and Waste Removal
Patients with kidney failure undergo dialysis, a medical process that mimics natural filtration using osmosis. In dialysis machines, a semipermeable membrane separates the patient’s blood from a specially formulated dialysate. Water and waste molecules move from the blood (high concentration) to the dialysate (low concentration), cleansing the blood.
This life-saving use of osmosis illustrates its role in modern medicine.
13. Saltwater Fish in Freshwater
Saltwater fish are adapted to live in high-solute environments. When placed in freshwater—a low-solute environment—water enters their cells rapidly via osmosis. The uncontrolled influx of water can cause their cells to swell, sometimes fatally.
This is why saltwater fish cannot survive in freshwater aquariums.
14. Freshwater Fish in Saltwater
Conversely, freshwater fish lose water quickly when placed in saltwater. The external high-solute environment draws water out of their bodies via osmosis, leading to rapid dehydration and cellular collapse.
Their inability to retain water in salty environments makes cross-habitat survival impossible without special adaptations.
15. Food Preservation with Pickling
Pickling preserves food through osmosis. Placing vegetables in a high-salt or high-sugar brine draws water out of microbial cells via osmosis. This dehydration inhibits bacterial growth and spoilage, keeping the food fresh for longer periods.
Pickles, jams, and salted meats rely on this osmotic principle to stay shelf-stable and safe.
Example Of Osmosis Summary Table
| Example | Description | Context |
| Plant Roots | Water moves from soil to roots | Plant growth |
| Fruits Shrinking | Water exits produce to dry air | Food storage |
| Raisins Plumping | Water enters raisins to balance sugar | Food science |
| Blood Cells (Hypertonic) | Water exits cells, causing shrinkage | Cell biology |
| Blood Cells (Hypotonic) | Water enters cells, causing bursting | Cell biology |
| Diabetes | Water moves from cells to blood, causing thirst | Human health |
| Cystic Fibrosis | Mucus draws water, causing dehydration | Human health |
| Kidneys | Water reabsorption concentrates urine | Human physiology |
| Bacteria | Nutrients enter via osmosis | Microbiology |
| Contact Lenses | Dry lenses draw water from tears | Everyday life |
| Dialysis | Waste moves from blood to dialysate | Medical treatment |
| Saltwater Fish | Water enters fish in freshwater | Marine biology |
| Freshwater Fish | Water exits fish in saltwater | Marine biology |
| Pickle Preservation | Salt draws water from food to preserve it | Food preservation |



Leave a Reply