What is Osmosis and How Does it Occur?
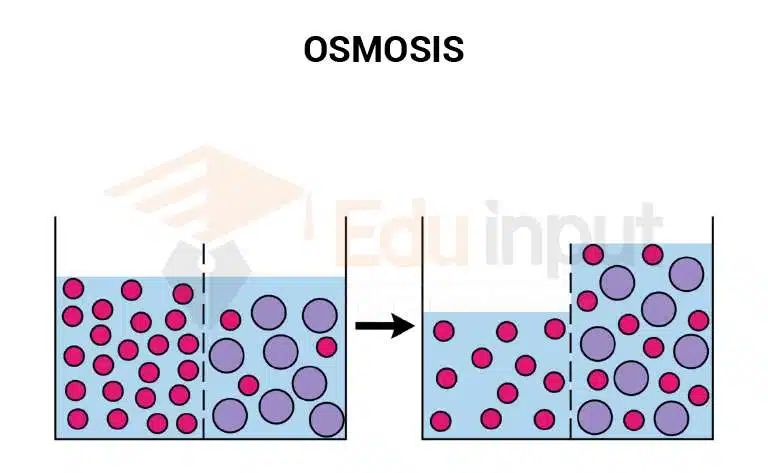
Osmosis is a biological process in which a solvent (water) moves from a high-concentration area to a low-concentration area. The term ‘osmosis’ was derived from the Greek words ‘Osmo’ meaning water and ‘potassos’ meaning power.
Osmosis is a natural phenomenon where water moves across a membrane from a region of higher concentration to a region of lower concentration. In nature, osmosis occurs constantly between cells and between organisms and their environment.
Osmosis is what makes our bodies work – it’s how we get rid of waste products and keep the body clean. Osmosis is also responsible for the movement of water from the roots to the leaves of a plant.
How Does Osmosis Occur?
Let’s assume that a solution is moving towards a low-concentration area and a high-concentration area. If the concentration gradient is very steep, then the solution will move quickly to the low-concentration area.
This means that the molecules of the solvent are moving more quickly to the lower concentration than the solute is moving to the higher concentration.
This diffusion of water is called osmosis. It is a natural phenomenon that is present in all living things. Osmosis is the basis of life and without this process, plants cannot grow and animals could not live.
Osmosis is a vital process that occurs in biological systems. This process is made possible by the presence of semipermeable membranes. In general, these membranes allow small molecules like oxygen, carbon dioxide, nitrogen, and nitric oxide to pass through, but they are impermeable to larger and polar molecules like ions, proteins, and polysaccharides.
The permeability of a given membrane depends on the solubility and charge of the molecules involved, as well as the size of the solute.
Water molecules travel through the cell by diffusing across the phospholipid bilayer. This process is facilitated by aquaporins, which are small transmembrane proteins. Osmosis is the primary means by which water is transported into and out of cells, and it is responsible for maintaining the turgor pressure of a cell.
Osmotic Pressure
When a solution and a pure solvent are separated by a membrane, and the solvent can pass through but the solute cannot, the solution will become more diluted. This happens because the solvent molecules move through the membrane into the solution. The process can be stopped by increasing the pressure on the solution to a specific amount, which is known as osmotic pressure.
Mechanism Of Osmosis
The mechanism that is responsible for driving osmosis is still being debated by scientists. Some say that it is the dilution of water by the solute. Others say that it is the solute’s attraction to water. Neither of these has been proven to be correct.
The mechanism that drives osmosis is commonly explained in biology and chemistry texts as either the dilution of water by a solute or the diffusion of water across a semipermeable membrane. Osmosis is when water molecules diffuse across a cell membrane from an area of high water concentration to an area of low water concentration.
This can happen either because of a solute’s attraction to water (resulting in less free water on the higher solute concentration side of the membrane and therefore net movement of water toward the solute) or by the fact that there is a higher concentration of solute particles on one side of the membrane than the other (which results in a lower concentration of water on the higher solute concentration side of the membrane and therefore a diffusion of water along a concentration gradient).



Leave a Reply