Beryllium-Discovery, Properties. And Applications
Beryllium, with the symbol ‘Be’ and atomic number 4, is a fascinating chemical element found in the Earth’s crust. This article delves into the key properties, history, and industrial applications of beryllium.
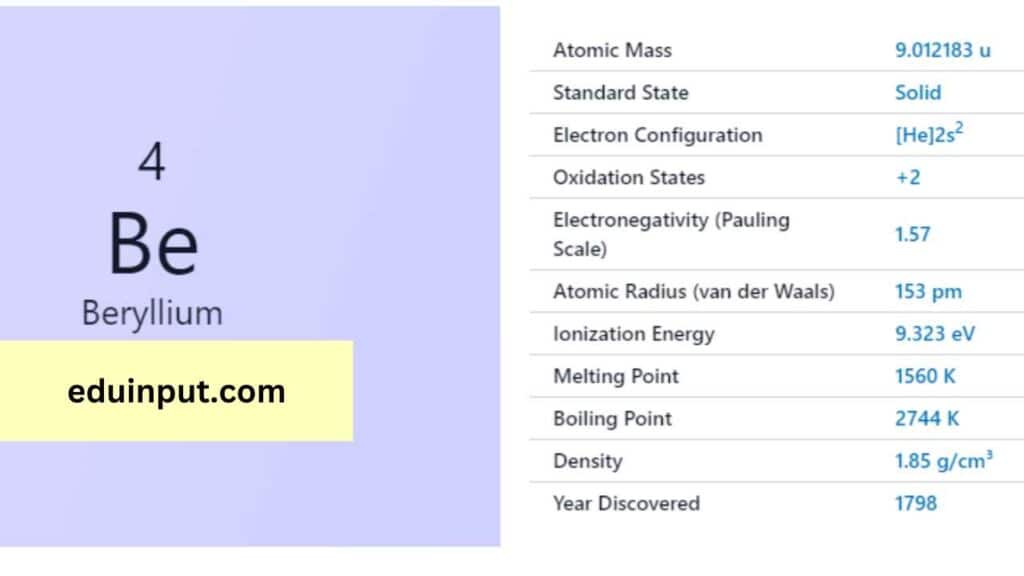
| Property | Value |
|---|---|
| Name | Beryllium |
| Symbol | Be |
| Atomic number | 4 |
| Relative atomic mass (Ar) | 9.0122 |
| Period in the periodic table | 2 |
| Group in the periodic table | 2 (Alkaline earth metal) |
| Period in the periodic table | 2 |
| Block in the periodic table | s |
| Shell structure | 2.2 |
Discovery
Beryllium was first discovered in 1798 by French chemist Louis Nicolas Vauquelin. However, it wasn’t until 1828 that two chemists, Friedrich Wöhler and Antoine Bussy, independently isolated pure beryllium through chemical processes.
Physical Properties
Beryllium is a relatively lightweight metal with a density of 1.85 g/cm³. It boasts a high melting point of 1,287°C and a boiling point of 2,970°C. Beryllium is exceptionally rigid and lightweight, making it an attractive element for specific applications.
Chemical Properties
Beryllium exhibits remarkable chemical properties. It is non-magnetic, resists oxidation when exposed to air, and is a good conductor of heat and electricity. Beryllium can also form strong covalent bonds with other elements, resulting in various interesting compounds.
Facts
- Beryllium has a single stable isotope, Be-9.
- It is used in the aerospace industry for lightweight structural components.
- Beryllium-copper alloys are used in electrical connectors and springs due to their high conductivity and durability.
- Beryllium is toxic when inhaled as dust or fumes, requiring strict safety precautions in its handling.
Applications
Beryllium’s unique properties find applications in several industries:
- Aerospace: Beryllium is utilized in aerospace components for its lightweight yet sturdy nature. It is often employed in satellites and high-performance aircraft.
- Electronics: Beryllium-copper alloys are favored for electrical connectors and springs due to their excellent conductivity and resistance to corrosion.
- Nuclear Industry: Beryllium is used as a neutron reflector in nuclear reactors and as a moderator in some nuclear reactions.
- X-ray Windows: Beryllium foils are used in X-ray windows, allowing the passage of X-rays while maintaining structural integrity.
- Scientific Research: Beryllium’s unique properties make it valuable in scientific research, particularly in X-ray equipment and particle physics experiments.





Leave a Reply