Alkali Metals-Properties, Electronic Configuration, and Periodic Trends
Alkali metals are a group of chemical elements found in the first column of the periodic table. These metals are highly reactive and are known for their ability to easily lose their outermost electron, resulting in the formation of positively charged ions.
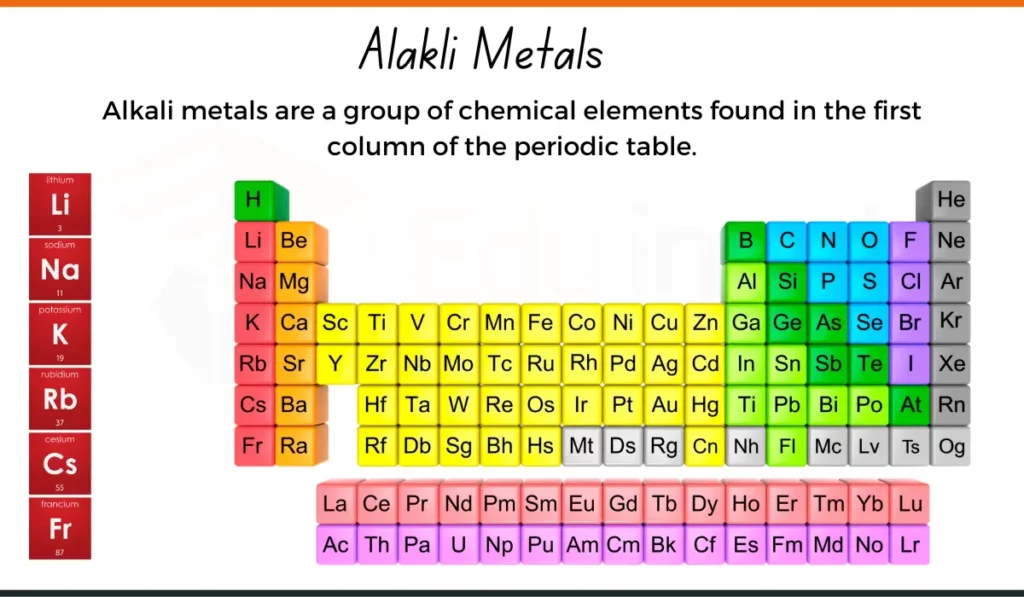
Read Examples of Alkali Metals
Alkali metals are called Aalaklis because they readily form alkaline solutions when they react with water. This reaction produces hydroxide ions, which are characteristic of alkaline substances.
The term “alkali” refers to a basic or alkaline substance, and these metals demonstrate this property vividly. Their ability to form strong bases when combined with water distinguishes them from other metals and contributes to their importance in various chemical and industrial processes.
Physical Properties of Alkali Metals
- Alkali metals are typically soft and can be easily cut with a knife. This softness increases down the group.
- They have relatively low densities compared to most other metals. The density generally increases down the group.
- Alkali metals have low melting and boiling points compared to other metals. These points decrease down the group.
- They exhibit high thermal conductivity, meaning they can efficiently transfer heat.
- Alkali metals have a shiny, metallic appearance, resembling silver.
- They are malleable, meaning they can be hammered or pressed into thin sheets, and ductile, meaning they can be drawn into wires.
- Alkali metals exhibit a characteristic metallic luster, reflecting light well.
Position in Periodic Table
Alkali metals reside in Group 1 of the periodic table, also known as the alkali metal group. This group includes elements such as lithium(Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They are placed on the far left side of the periodic table, preceding the alkaline earth metals.)
Occurrence
Alkali metals are relatively abundant in nature. They are typically found in compound form rather than as pure elements. Sodium is widely distributed in minerals such as halite (rock salt) and in seawater. Sodium is also present in common salt or sodium chloride.
Potassium is present in minerals like feldspar, carnallite, and sylvite, as well as in plant and animal tissues. Lithium occurs in various minerals, including spodumene, lepidolite, and petalite, and is also found in small amounts in seawater and certain mineral springs.
Rubidium and cesium are primarily obtained from the minerals lepidolite, pollucite, and carnallite. Francium is extremely rare and radioactive, with its occurrence limited to trace amounts in uranium and thorium ores.
Electronic configuration
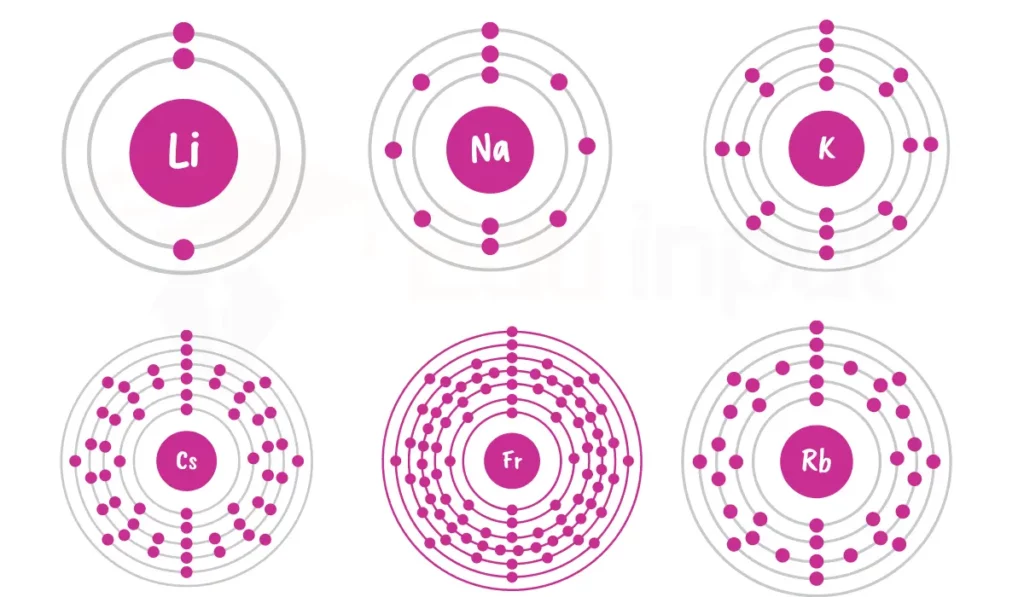
The general electronic configuration of alkali metals is [Noble gas] ns¹. Here are the electronic configurations of different alkali metals:
| Element | Atomic Number | Electron Configuration |
| Lithium | 3 | 1s² 2s¹ |
| Sodium | 11 | 1s² 2s² 2p⁶ 3s¹ |
| Potassium | 19 | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ |
| Rubidium | 37 | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s¹ |
| Cesium | 55 | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s¹ |
| Francium | 87 | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p⁶ 7s¹ |
General Trends in Periodic Table
Alkali metals exhibit several general trends in the periodic table:
- Atomic Size – Alkali metals have the largest atomic sizes in their respective periods due to the presence of only one electron in their outermost shell. As you move down the group, atomic size increases due to the addition of energy levels.
- Reactivity – Alkali metals are highly reactive due to their tendency to lose an electron easily. This reactivity increases down the group, with francium being the most reactive alkali metal.
- Melting and Boiling Points – Alkali metals have relatively low melting and boiling points compared to other metals, and these points decrease down the group.
- Density -Alkali metals are less dense compared to transition metals, and their density increases down the group.
- Ionization Energy – Alkali metals have low ionization energies, meaning it requires relatively little energy to remove their outermost electron. Ionization energy decreases down the group.



Leave a Reply