Chromium-Discovery, Properties, And Applications
Chromium is a chemical element with the symbol ‘Cr’ and atomic number 24. It is a hard, silvery-grey, lustrous metal that belongs to the transition metal group of the periodic table. Chromium has unique properties that make it useful in various applications, including its corrosion resistance, hardness, and ability to form colorful compounds.
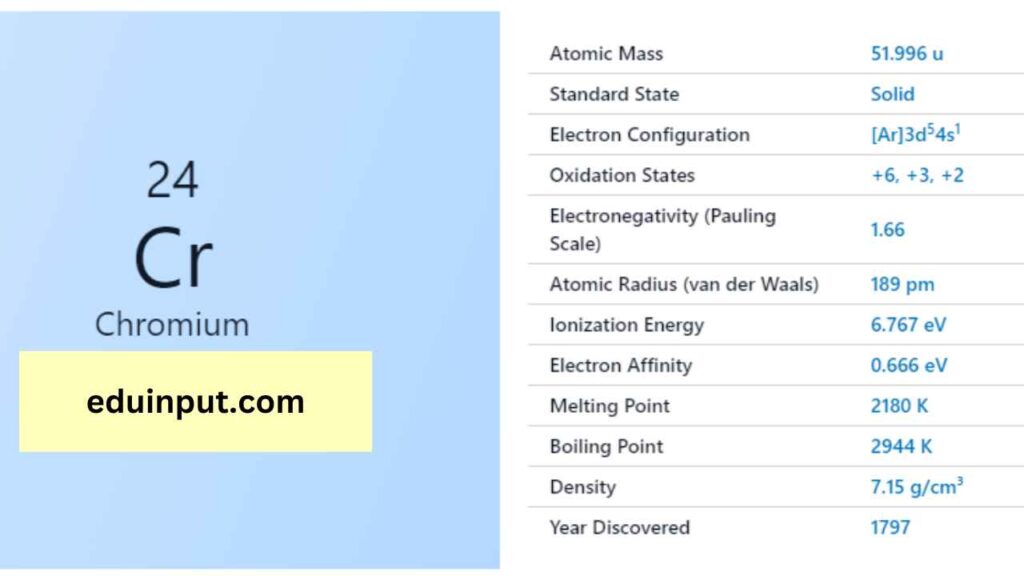
| Group in the periodic table | Value |
| Name | Chromium |
| Symbol | Cr |
| Atomic number | 24 |
| Relative atomic mass (Ar) | Period in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery metallic |
| Classification | Metallic |
| Group in periodic table | 6 |
| Group name | (none) |
| Block in the periodic table | 4 |
| Block in periodic table | d |
| Shell structure | 2.8.13.1 |
| CAS Registry | 7440-47-3 |
Discovery
Chromium was discovered by Louis-Nicholas Vauquelin, a French chemist, in 1797. He found the element in a red lead mineral known as crocoite, which he analyzed and isolated chromium from.
Physical Properties
Chromium is a hard, brittle, and lustrous metal with a high melting point of 1,907°C and a boiling point of 2,671°C. It has a high density of 7.14 g/cm³ and a Mohs hardness of 8.5, making it a very hard metal. Chromium is also highly resistant to corrosion due to its ability to form a thin oxide layer on its surface.
Chemical Properties
Chromium has an electron configuration of [Ar]3d^54s^1 and can form compounds in various oxidation states, ranging from -2 to +6. Chromium has a unique property of forming colorful compounds, which is used in the production of pigments for paints, inks, and plastics.
Facts
- Chromium is named after the Greek word ‘chroma’ meaning color, due to its ability to form colorful compounds.
- Chromium is used in the production of stainless steel, which is highly resistant to corrosion.
- Chromium is an essential nutrient for humans, playing a role in glucose metabolism.
Applications
Chromium is widely used in various applications due to its unique properties. Its ability to form colorful compounds is used in the production of pigments for paints, inks, and plastics. Chromium is also used in the production of stainless steel, which is highly resistant to corrosion, making it ideal for applications in the chemical, food, and medical industries. Chromium is also an essential nutrient for humans and is used in supplements to regulate blood sugar levels.
Chromium is a hard, silvery-grey, lustrous metal with unique properties that make it useful in various applications. Its corrosion resistance, hardness, and ability to form colorful compounds have made it a valuable material in the production of pigments, stainless steel, and supplements for human health.






Leave a Reply