Types of Hemoglobin With Structure and Functions
Hemoglobin Definition
Hemoglobin is a protein found in red blood cells that helps carry oxygen throughout the body.
What is Hemoglobin?
Hemoglobin is a respiratory carrier that performs a two-way function. It carries oxygen from the lungs to the body tissues and also helps in transporting carbon dioxide back to the lungs.
Hemoglobin is a shortened term derived from the medical word “haemato-globulin,” which originates from the Greek language. It roughly translates to “blood grains” and refers to the protein present in red blood cells.
The abbreviation for hemoglobin is “Hb”.
Hemoglobin Levels
The levels of hemoglobin in males and females can vary.
- For adult males normal hemoglobin levels are typically between 13.5 to 16.5 grams per deciliter (g/dL).
- For adult females normal hemoglobin levels are usually between 12.0 to 14.5 grams per deciliter (g/dL).
For males, a hemoglobin level below 13.5 g/dL and for females, a level below 12.0 g/dL is generally considered low and may require further investigation and medical attention.
Structure Of Hemoglobin
Hemoglobin is a conjugated protein. It is made up of globin and heme. Globin is the protein part, and heme is the non-protein part. Hemoglobin is a tetrameric allosteric protein, meaning that it is made up of four subunits.
The two alpha subunits and the two beta subunits are held together by non-covalent bonds.

Structure Of Globin
The globin subunits are made up of amino acids. Each alpha subunit has 141 amino acids, and each beta subunit has 146 amino acids. The amino acids in the globin subunits form a quaternary structure, which is the overall shape of the protein.
However, some authors only consider hemoglobin to be made up of two identical dimmers. Each D-chain has 141 amino acids while the E-chain has 146 amino acids.
Combined, HbA1 has a total of 574 amino acid residues. The four subunits of hemoglobin are held together by non-covalent hydrophobic, ionic, and hydrogen bonds. Lastly, each subunit contains a heme group.
Structure Of Heme
The heme group is a non-protein part of hemoglobin. It is a porphyrin ring with an iron atom at the center. The iron atom is what gives hemoglobin its red color. The heme group is also what allows hemoglobin to bind oxygen.
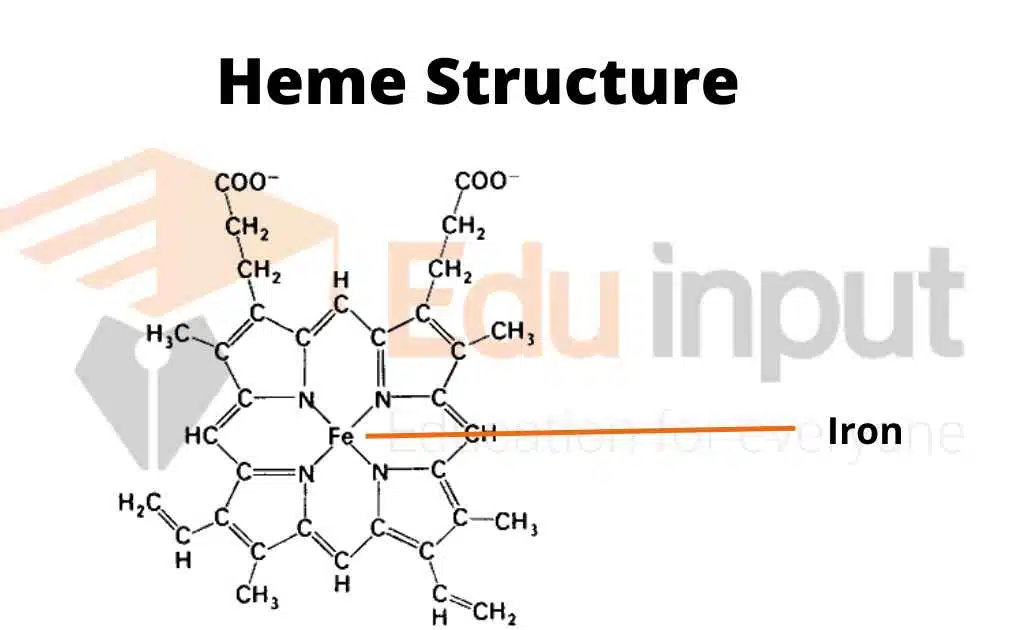
Binding Sites
Hemoglobin has four binding sites for oxygen. Each binding site is located on a heme group. When oxygen binds to hemoglobin, it changes the shape of the protein.
This change in shape makes it easier for hemoglobin to bind to more oxygen.
Shape and Color
Hemoglobin is a tetrahedral protein. This means that it has four faces that are all the same shape.
Hemoglobin is also red in color. This is because of the iron atom in the heme group.
Types of Hemoglobin
Different types of hemoglobin work together to ensure efficient oxygen transport and maintain the oxygen-carrying capacity of the blood.
In adults, there are three main types of Hb found in the blood:
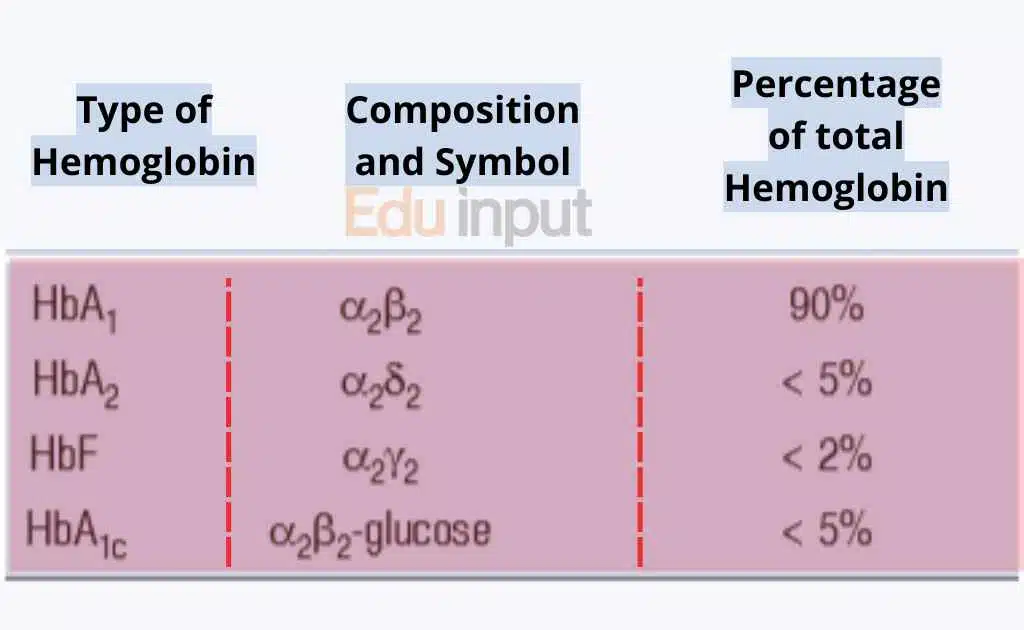
Hemoglobin A (HbA)
This is the most common type of hemoglobin in adults, making up about 95-98% of the total hemoglobin. Hemoglobin A is composed of two alpha-globin chains and two beta-globin chains.
Its main function is to transport oxygen from the lungs to body tissues. It binds with oxygen in the lungs and forms oxyhemoglobin. Then it releases oxygen to cells.
Additionally, it helps transport a small amount of carbon dioxide back to the lungs for exhalation, a waste product of cellular metabolism.
Hemoglobin A2 (HbA2)
Hemoglobin A2 is the second most prevalent type of hemoglobin in adults, accounting for about 2-3% of the total hemoglobin. It is made up of two alpha-globin chains and two delta-globin chains.
Its exact role is not entirely clear, but it may play a backup or compensatory function when levels of Hemoglobin A are decreased or impaired.
Hemoglobin F (HbF)
Hemoglobin F is mainly found in fetuses during development. Typically it makes up less than 1% of the total hemoglobin. It is made up of two alpha-globin chains and two gamma-globin chains.
Its primary function is to facilitate the transfer of oxygen from the mother’s blood to the fetus through the placenta. Hemoglobin F has a higher affinity for oxygen compared to Hemoglobin A.
It allows it to efficiently extract oxygen from the mother’s bloodstream and deliver it to the developing fetus.
After birth, the production of Hemoglobin F decreases gradually. And Hemoglobin A becomes the predominant type of hemoglobin in the blood.
Small amounts of Hemoglobin F may persist in adults, but its function becomes less significant after the neonatal period.
Hemoglobin Variants
Hemoglobin variants refer to different types of hemoglobin molecules that result from various combinations of its subunits or mutations. They can be a natural part of normal embryonic and fetal development.
Over 1000 naturally occurring human hemoglobin variants have been identified. These variants are characterized by single amino acid substitutions throughout the molecule.
There are many different types of hemoglobin variants. Some of the most common types include:
HbS (Sickle Cell Anemia)
A mutation that changes hemoglobin’s shape. It causes serious blood disorder with deformed red blood cells.
HbC (Cooley’s Anemia)
A mutation altering hemoglobin’s amino acid sequence. It leads to milder thalassemia with anemia.
HbE (E Thalassemia)
A mutation changing hemoglobin’s amino acid sequence, causing mild thalassemia with mild anemia.
HbD (D Thalassemia)
A mutation modifying hemoglobin’s amino acid sequence. It results in mild thalassemia with mild anemia.
Function Of Hemoglobin
Hemoglobin is responsible for some key functions related to respiration.
- Hemoglobin is largely responsible for the oxygen transport from the lungs to the tissues.
- Hemoglobin also helps to transport CO2 from the tissues to the lungs. This is how we get rid of CO2 and protons from our bodies.
- Hemoglobin plays a role in metabolism. Metabolism is the process by which the body converts food into energy.
How Do O2 Binds To Hemoglobin?
One molecule of hemoglobin can bind with up to four molecules of oxygen. This is in contrast to myoglobin (with one Heme) which can bind with only one molecule of oxygen. In other words, each Heme moiety can bind with one O2.



Leave a Reply