Synaptic Ion Channels-Structure, Types, and Importance
What are Synaptic Ion Channels?
Synaptic ion channels are pore-forming membrane proteins located in the presynaptic and postsynaptic terminals of chemical synapses. They allow the flow of ions across the neuron’s membrane in response to neurotransmitters, voltage changes, and other signals.
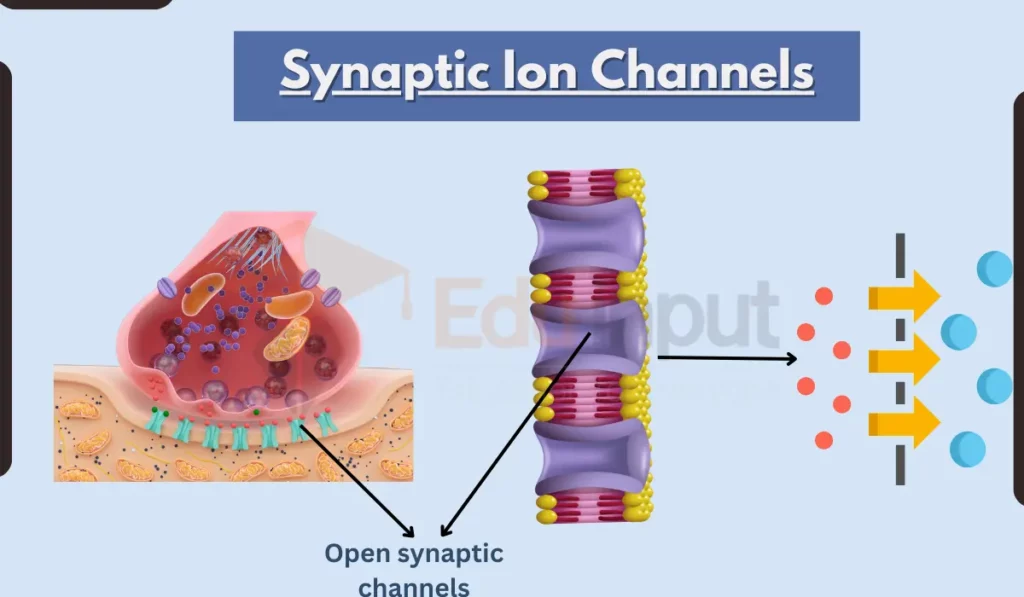
The main types of ions that pass through synaptic channels are sodium, potassium, calcium, and chloride.
Synaptic ion channels play important roles in synaptic transmission and plasticity. When an action potential reaches the axon terminal, it causes voltage-gated calcium channels to open. This allows calcium to enter the neuron and trigger the release of neurotransmitter-filled vesicles into the synaptic cleft.
The neurotransmitter then diffuses and binds to receptors on the postsynaptic membrane, opening ligand-gated ion channels that allow ions to flow into or out of the postsynaptic cell. This ion flow produces excitatory or inhibitory postsynaptic potentials.
Why are Synaptic Ion Channels Important?
Synaptic ion channels are essential for the propagation of electrical signaling between neurons. If these channels did not allow rapid ion flux at synapses, neurons would not be able to communicate and coordinate activity.
In addition, the diverse types of synaptic ion channels present on neurons determine their excitability and signaling properties. The complement of channels expressed influences the neuron’s firing patterns, response timescale, and ability to strengthen or weaken connections.
Synaptic ion channels also underlie key functions like working memory, movement control, and sensation.
Overall, the carefully regulated opening and closing of these channels enables complex information processing in the brain.
Types of Synaptic Ion Channels
There are multiple classes of synaptic ion channels:
1. Voltage-gated channels
These open and close in response to changes in membrane voltage. At axon terminals, voltage-gated Ca2+ channels mediate neurotransmitter release. In dendrites, voltage-gated Na+, K+, and Ca2+ channels shape postsynaptic potentials.
2. Ligand-gated channels
These ionotropic receptors open when bound by a chemical messenger like a neurotransmitter. Major types include NMDA, AMPA, nicotinic acetylcholine, GABA-A, and 5-HT3 receptors.
3. Leak channels
These are constantly open at rest, allowing steady ion flux. They establish the resting membrane potential and input resistance of neurons.
4. Metabotropic receptors
These G protein-coupled receptors indirectly regulate ion channels through second messenger cascades when activated by neurotransmitters.
Structure of Synaptic Ion Channels
Synaptic ion channels are specialized membrane proteins that allow ions to flow across the cell membrane in response to various stimuli, such as neurotransmitters, voltage changes, or mechanical force. They play a vital role in synaptic signaling, which is the process by which neurons communicate with each other.
While synaptic ion channels have diverse structures, they all share some common architectural features:
1. Pore domain
This domain forms the actual ion-permeable channel that crosses the membrane. It contains a selectivity filter that determines which ions can pass through.
2. Gate domain
This domain controls the opening and closing of the channel. Ligand-gated channels have a physical gate that is opened by the binding of a neurotransmitter. Voltage-gated channels have a more complex electromechanical coupling mechanism that opens the channel in response to changes in membrane voltage.
3. Regulatory domains
These domains sense stimuli that open or close the channel. Ligand-binding domains and voltage sensors are found in ligand- and voltage-gated channels, respectively.
4. Auxiliary subunits
These subunits associate with the pore-forming subunits and modify channel properties like conduction and gating.
Functions of Synaptic Ion Channels
Synaptic ion channels carry out several key functions:
- Mediating synaptic transmission – The coordinated opening of presynaptic voltage-gated Ca2+ channels and postsynaptic ligand-gated channels allows rapid signaling between neurons.
- Shaping postsynaptic potentials – Ion flux through AMPA, NMDA, and GABA receptors generates excitatory and inhibitory postsynaptic potentials with defined time courses.
- Synaptic integration – Spatial and temporal summation of inputs arriving on dendrites is governed by the interplay between ligand- and voltage-gated channels.
- Synaptic plasticity – Activity-dependent changes in synaptic strength rely on ion channels like NMDA receptors that flux Ca2+ and trigger intracellular signaling cascades.
- Neurotransmitter release – Presynaptic voltage-gated channels, particularly Ca2+ channels, control the amount of neurotransmitter release in an activity-dependent manner.
Synaptic Ion Channels in Synaptic Plasticity
Synaptic ion channels play indispensable roles in mediating synaptic plasticity, the ability of synaptic connections to strengthen and weaken. This neuronal plasticity enables learning, memory formation, and development.
Key mechanisms like long-term potentiation (LTP) and long-term depression (LTD) depend on the ion flux through postsynaptic NMDA receptors and metabotropic glutamate receptors. These receptors alter intracellular Ca2+ levels and activate downstream signaling pathways that increase or decrease synaptic AMPA receptors.
Presynaptic ion channels that regulate neurotransmitter release, like voltage-gated Ca2+ channels, also modulate synaptic plasticity. The complex coordination of pre- and postsynaptic ion channel activity drives persistent changes in synaptic strength.
Synaptic Ion Channels in Neurological Disorders
Dysfunction of synaptic ion channels has been implicated in many neurological diseases:
- Epilepsy – Altered function of voltage-gated Na+ and Ca2+ channels leads to neuronal hyperexcitability and seizures.
- Chronic pain – Peripheral neuron sensitization involves changes in various voltage-gated channels. NMDA receptor activation also mediates central pain plasticity.
- Neurodegenerative diseases – Imbalances in synaptic excitation and inhibition underlie cognitive decline in Alzheimer’s. NMDA receptor dysfunction may contribute to excitotoxicity.
- Autism spectrum disorders – Evidence suggests altered synaptic ion channel expression and impaired plasticity mechanisms play a role.
- Mood disorders – Disrupted ion channel activity may contribute to abnormal synaptic transmission and plasticity underlying depression and bipolar disorder.
Drug Targeting of Synaptic Ion Channels
The critical physiological roles of synaptic ion channels make them important targets for pharmacological agents to treat CNS and pain disorders:
- Benzodiazepines act as positive allosteric modulators of GABA-A receptors, enhancing inhibition to produce anxiolytic and anticonvulsant effects.
- Many antidepressants and antipsychotics block serotonin, norepinephrine, and dopamine transporters – indirect modulation of ligand-gated channels.
- Voltage-gated Na+ channel blockers like lamotrigine help stabilize neuronal excitability in bipolar disorder and epilepsy.
- NMDA receptor antagonists offer neuroprotection in stroke and may help treat depression and chronic pain. However, they carry side effects like memory impairment.

 written by
written by 


Leave a Reply