Potassium-Discovery, Properties, and Applications
Potassium is a chemical element with the symbol ‘K’ and atomic number 19. It is an alkali metal and is the seventh most abundant element in the Earth’s crust. Potassium plays an essential role in various biological processes and is necessary for the proper functioning of the human body.
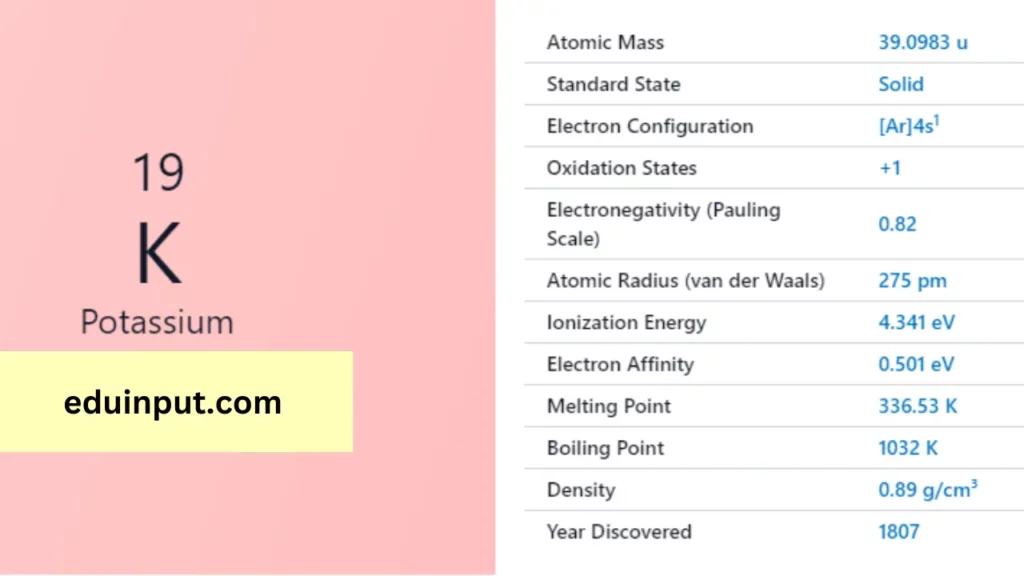
| Property | Value |
| Name | Potassium |
| Symbol | K |
| Atomic number | 19 |
| Relative atomic mass (Ar) | 39.0983 (1) |
| Standard state | Solid at 298 K |
| Appearance | Silvery white |
| Classification | Metallic |
| Group in periodic table | 1 |
| Group name | Alkali metal |
| Block in the periodic table | 4 |
| Group in the periodic table | s |
| Shell structure | 2.8.8.1 |
| CAS Registry | 7440-09-7 |
Discovery
Potassium was discovered by Sir Humphry Davy in 1807. He isolated it from the mineral potash, which is a naturally occurring compound that contains potassium. Davy named the element potassium after the English word ‘potash’.
Physical Properties
Potassium is a soft, silvery-white metal that can be easily cut with a knife. It has a low melting point of 63.38°C and a boiling point of 759°C. Potassium is highly reactive and can ignite when exposed to air or water.
Chemical Properties
Potassium is a highly reactive metal and forms compounds with a variety of elements. It reacts vigorously with water to produce potassium hydroxide and hydrogen gas. Potassium also reacts with oxygen to form potassium oxide and halogens to form various salts.
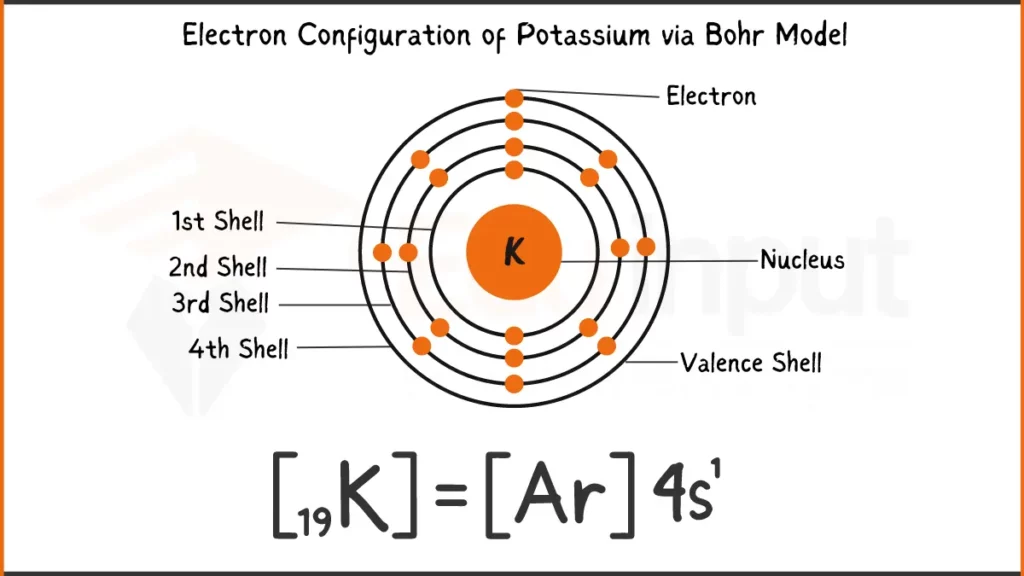
Electronic Configuration of Potassium
Potassium (K) possess 19 electrons. Its configuration can be written as [Ar] 4s¹. It has one electron in the outermost 4s subshell. This single electron in the 4s subshell makes Potassium a valence electron, meaning it readily participates in bonding with other elements.
Electronic Configuration of Potassium via Bohr Model
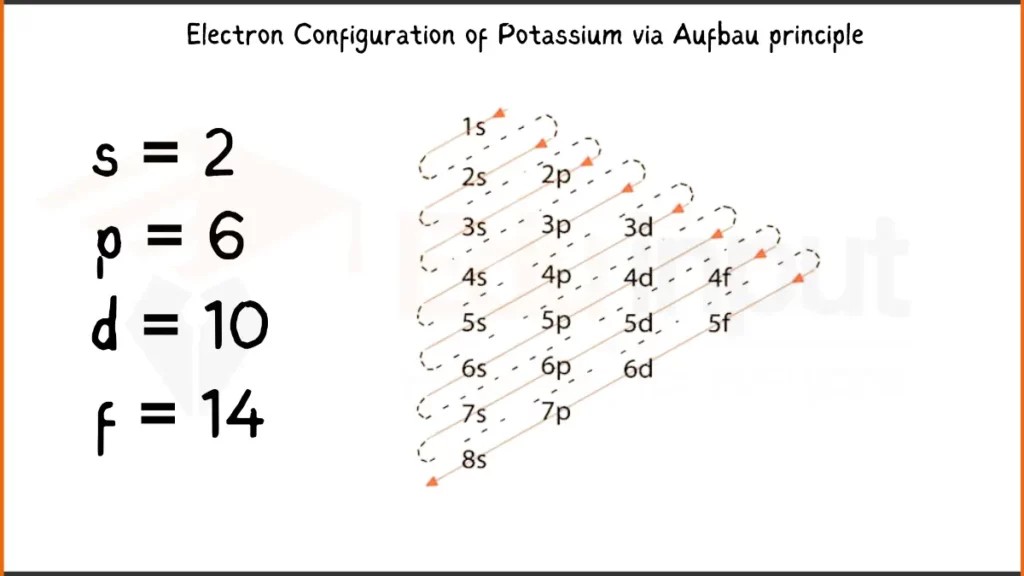
Electronic Configuration of Potassium via Aufbau Principle
Facts
- Potassium is essential for the proper functioning of the human body. It helps to regulate blood pressure, maintain fluid balance, and support muscle and nerve function.
- Bananas are a rich source of potassium and are often recommended as a dietary source of this element.
- Potassium is commonly used in fertilizers to improve plant growth and yield.
- Potassium is also used in the manufacture of soaps, glass, and other industrial products.
Applications
Potassium has a wide range of applications in various industries. Some of the major applications of potassium include:
- Agriculture: Potassium is used as a key ingredient in fertilizers to improve soil fertility and plant growth.
- Food industry: Potassium is used as a preservative in food products to extend their shelf life.
- Pharmaceuticals: Potassium supplements treat and prevent potassium deficiencies in the human body.
- Chemical industry: Potassium is used as a reagent in various chemical reactions to produce a range of products.
Potassium is an essential element for life and plays a vital role in various biological and industrial processes. Its unique physical and chemical properties make it a valuable element with many applications.





Leave a Reply