Terbium-Discovery, Properties, And Applications
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that belongs to the lanthanide series. Terbium was discovered in 1843 by Swedish chemist Carl Gustaf Mosander, who first separated it from a mixture of rare earth elements.
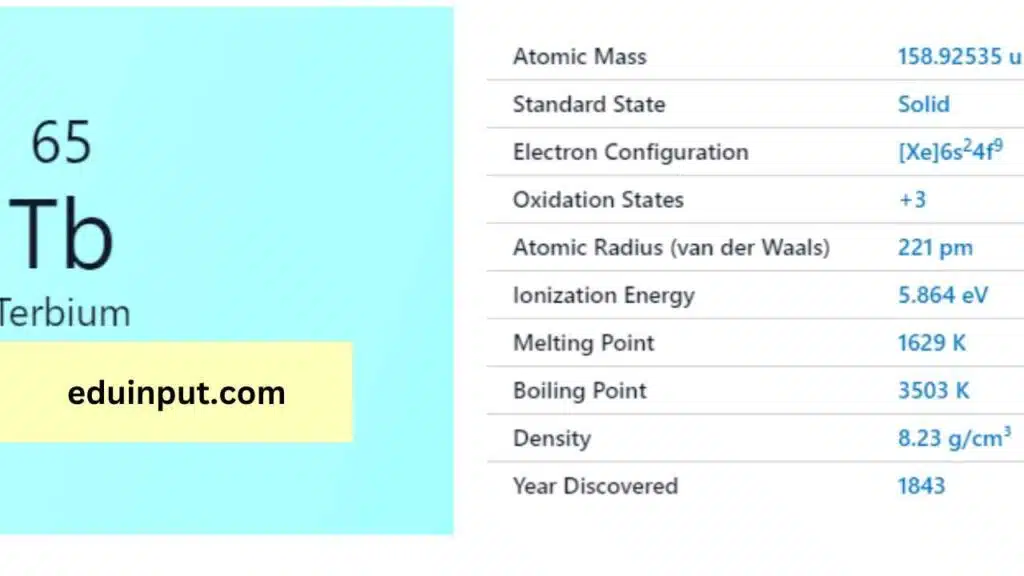
| Property | Value |
| Name | Terbium |
| Symbol | Tb |
| Atomic number | 65 |
| Relative atomic mass (Ar) | Period in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery white |
| Classification | Metallic |
| Group in periodic table | |
| Group name | Lanthanoid |
| Block in the periodic table | 6 (lanthanoid) |
| Group in the periodic table | f |
| Shell structure | 2.8.18.27.8.2 |
| CAS Registry | 7440-27-9 |
Physical Properties
Terbium is a soft, silvery-white metal that is relatively stable in air. It is malleable and ductile and has a melting point of 1,356°C and a boiling point of 3,230°C. It is paramagnetic, meaning that it is weakly attracted by a magnetic field.
Chemical Properties
Terbium is a reactive metal that slowly oxidizes in air. It reacts with water to form terbium hydroxide and hydrogen gas. It also reacts with acids to form salts, such as terbium chloride (TbCl3) and terbium nitrate (Tb(NO3)3).
Facts
- Terbium is named after the village of Ytterby in Sweden, where the rare earth mineral gadolinite was first discovered.
- Terbium is used in the production of green phosphors for television and computer screens.
- Terbium is also used in lasers, fuel cells, and nuclear reactors.
Applications
Terbium is most commonly used in the production of green phosphors for use in television and computer screens. It is also used in other phosphors, such as those used in fluorescent lamps, and in magneto-optical storage devices. Terbium is also used in the production of lasers and as a dopant in materials used in solid-state devices.
Terbium is a rare earth metal that has important applications in technology, particularly in the production of green phosphors for television and computer screens.
Its unique chemical and physical properties make it a valuable addition to various industries, and ongoing research may uncover new applications for this element in the future.





Leave a Reply