Difference Between Bioavailability and Bioequivalence
December 18, 2023
Table of Contents
Key Difference
Bioavailability refers to the rate and extent to which a drug is absorbed and becomes available at the intended site of action. Bioequivalence, on the other hand, indicates that two drugs have similar bioavailability and produce the same effect at the site of action, implying that they can be interchangeable.
Comparative Analysis
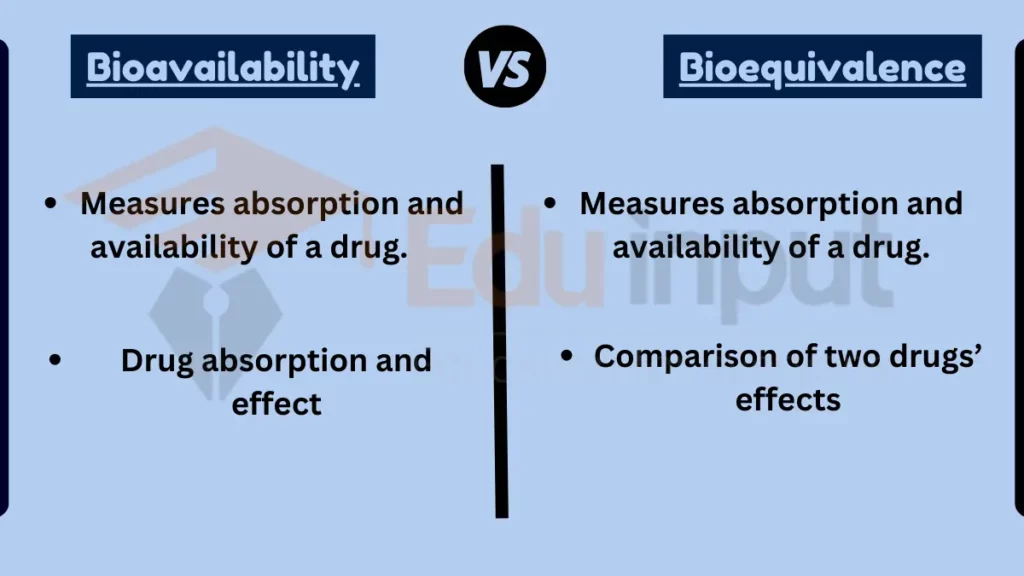
- Focus:
- Bioavailability: Measures absorption and availability of a drug.
- Bioequivalence: Compares two drugs’ effects and absorption rates.
Table Summary of Bioavailability vs Bioequivalence
| Feature | Bioavailability | Bioequivalence |
|---|---|---|
| Focus | Drug absorption and effect | Comparison of two drugs’ effects |
Bioavailability and bioequivalence are crucial concepts in pharmacology, determining how drugs work in the body and how they can be substituted for each other.
File Under:




Leave a Reply