What is Adiabatic Process-Definition, Types, And Example
A process in which no heat enters or leaves the system is called an adiabatic process.
Adiabatic Process
The adiabatic process is the process where the working fluid undergoes slow compression or expansion without any significant change in temperature. But the process in which the temperature of the system remains constant is called the isothermal process. This process can be used in different fields of engineering and science like refrigeration and air conditioning.
The adiabatic process is the process of the working fluid in which the temperature of the working fluid remains almost constant during the process. In this process, there is no heat exchange between the working fluid and the surroundings. Since no heat enters or leaves the system, so Q = 0
From the first law of thermodynamics
Q= Δ U + W
W= -ΔU
This equation can be expressed in two ways.
- Adiabatic Compression
- Adiabatic Expansion
The graph between P and V is called the PV diagram. The curve obtained is called an “Adiabatic”.
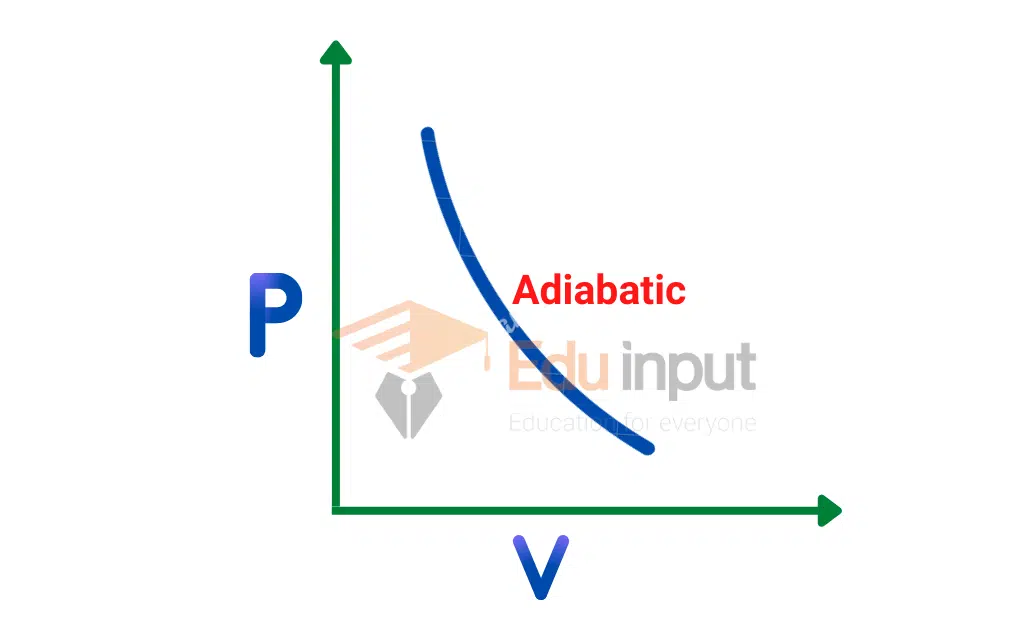
This curve is represented by the equation;
PVγ=Constant
γ=Cp/Cv=molar specific heat at constant Pressure/ molar specific heat at constant volume
Adiabatic Compression
If U = – ΔW
If work is done on the gas, then the temperature of the gas increases. As a result, the internal energy of the System increases. It is known as Adiabatic Compression.
An adiabatic compressor is used to increase the pressure of a gaseous fluid. The compression process is performed with the aid of a piston inside a cylinder filled with gas. The gas is compressed by moving the piston towards the end of the cylinder.
Adiabatic Expansion
If W = -ΔU
If work is done by the gas, then it does it at the cost of its own internal energy. As a result, the internal energy of the system decreases. It is known as Adiabatic Expansion.
An adiabatic expansion is also referred to as a Joule-Thomson Expansion. It is a process that reduces the temperature of a gaseous fluid. The temperature decreases due to the loss of heat to the surroundings. This process is carried out by increasing the internal pressure of a container, thereby pushing the gas out of it.
Adiabatic Process Examples:
- The rapid escape of air from a burst tire
- The rapid expansion and compression of air through which a sound wave is passing.
- Cloud formation in the atmosphere
Types of Adiabatic Process
There are different types of adiabatic process
Adiabatic Cooling
Adiabatic cooling is a process where heat is removed from a material by its surroundings without changing the temperature of the material. Adiabatic cooling is carried out using the principles of an adiabatic expansion.
Adiabatic Heating
Adiabatic heating is a process where heat is added to a material by its surroundings. This process can be achieved by compressing a material with the aid of an adiabatic expansion.
Adiabatic Refrigeration
Adiabatic refrigeration is also known as a Joule-Thomson Expansion. It is a process that reduces the temperature of a gaseous fluid without changing the volume of the gas.
Adiabatic Heat Pump
An adiabatic heat pump is also known as a Carnot Cycle. It is a device that transfers heat from one place to another, but it does not change the temperature of the heat source or the sink. This is a reversible process.




Leave a Reply