What Is Energy?-Definition, Types, And The laws of energy transformations
The capacity to do work is called energy. Work is the transfer of energy.
What Is Energy?
Energy can also be transformed. For example, a plant can transform solar energy into chemical energy. The tidal energy which comes from waves is converted into electric current which is electrical energy. The plant can then be burned in a steam generator. Thus its energy is transformed into the energy of motion. Energy has many forms
- The heat from a furnace
- The sound of a jet plane
- The electric current that lights a bulb
- The radioactivity in a heart pacemaker (CT scan machine)
- The pull of a magnet
- The non-conventional energy sources that produce electrical energy
Units of energy
The SI unit of energy is the joule. Energy is also measured in terms of heat production. The study of energy is called thermodynamics. The commonly used unit for measuring heat in an animal is the kilocalorie or calorie.
What is Calorie?
A kilocalorie (Kcal) is the amount of heat required to raise the temperature of 1 kg of water by 1° C. It is equal to 1,000 calories. A daily intake of energy for an average person is approximately 2,000 to 2,500 kcal.
There are some more units of energy.
| British thermal unit (BTU) | Kilowatt-hour (kWh) |
| Calorie | Electronvolts (eV) |
| Rydberg units | Hartree (the atomic unit of energy) |
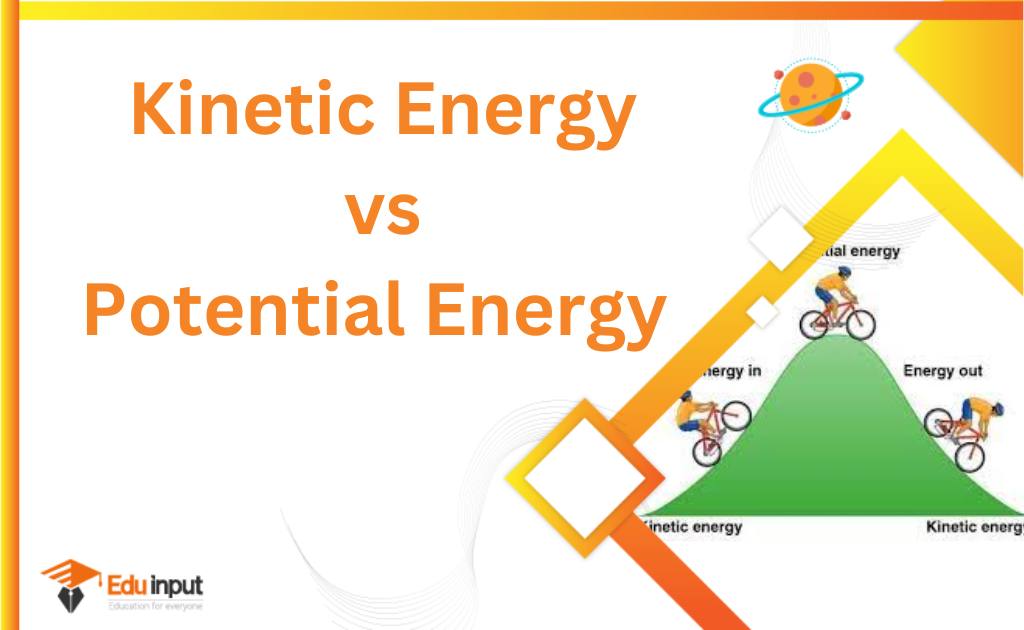
Types of Energy
Energy exists in two states
1. Kinetic energy
2. Potential energy
Kinetic energy
The energy possessed by a body due to its motion is called kinetic energy.
Mathematically
K.E= 1/2mv2
m= mass of the body
V=velocity of the body.
K.E. is a scalar quantity since K.E=1/2mv2
Unit of K.E. is the joule.
K.E. is directly proportional to the mass of the object.
K.E. is directly proportional to the square of the velocity of the object,i.e if v is doubled then K.E becomes four times.
If P is momentum then energy is related to momentum as
E= P2/2m
Potential energy:
The energy possessed by a body due to its position in a force field e.g gravitational field or due to its constrained state is called Potential energy.
Gravitational potential energy:
The potential energy due to a gravitational field near the surface of the Earth at a height ‘h’ is called Gravitational Potential energy.
Mathematically
P.E = m g .h
The gravitational potential energy is always to find out with reference to a point whose potential energy is taken as zero.
The two points taken as zero reference level of gravitational P.E. are
- The surface of Earth
- A point at infinity from the Earth
Elastic potential energy:
The energy stored in a compressed spring due to its compressed or stretched state is called Elastic Potential energy.
Mathematically,
P.E= 1/2KX2
K = spring constant of Hook’s Law
x= extension
Electric potential energy:
The energy acquired by a charge as it passes through some potential difference is called electric potential energy.
Mathematically,
P.E= Vq
q=charge
The laws of energy transformations
There are two laws of thermodynamics:
The first law of thermodynamics
the first law of thermodynamics is also called the law of energy conservation. This law states that energy can only be transformed from one form to another, it can neither be created nor e destroyed For example, electrical energy passes through a hot plate to produce heat energy. But energy can never be lost or created. Therefore, in the universe, the total amount of energy remains constant.
The second law of thermodynamics
The second law of thermodynamics states that all objects in the universe tend to become more disordered and that the total amount of disorder in the universe is continually increasing. The measure of this degree of disorganization is called entropy. For example, natural gas is burnt on a stove. Potential chemical energy is stored in the bonds of the gas molecules. This energy is converted into light (the blue flame) and heat. Some of the heat energy can be used to boil water on the stove.
Some energy is lost in the kitchen. This energy is no longer available to do work. This unusable energy increased entropy.





Leave a Reply