Isothermal Process | Isothermal Process and Boyle’s Law
An isothermal Process is defined as A process in which the temperature of the system remains constant is called an Isothermal process.
What is Isothermal Process?
According to the first Law of thermodynamics, there are two types of processes that define the relationship between work and heat.
- isothermal process
- adiabatic process
The isothermal process is the process where the heat transfer rate stays almost constant while the temperature varies. So, it is the process of keeping the desired temperature constant. But we are not discussing this type of process.
When the process of the heat transfer is constant then it is called the isothermal process.
Isothermal process and Boyle’s Law
For an Isothermal process, Boyle’s law can be applied
Suppose during an isothermal change, pressure and volume change from P₁V₁ to P2 V2. Then according to Boyle’s law,
P₁V₁ = P₂V₂
Since the temperature of the system remains constant, So there will be no change in the internal energy, i.e. ΔU = 0.
Hence, from the first law of thermodynamics
Q= Δ U +W
Q = W
It means that when a gas expands under the isothermal process and does external work W, an amount of heat Q has to be supplied. The graph between P and is called the PV diagram. The curve obtained is called an Isotherm.
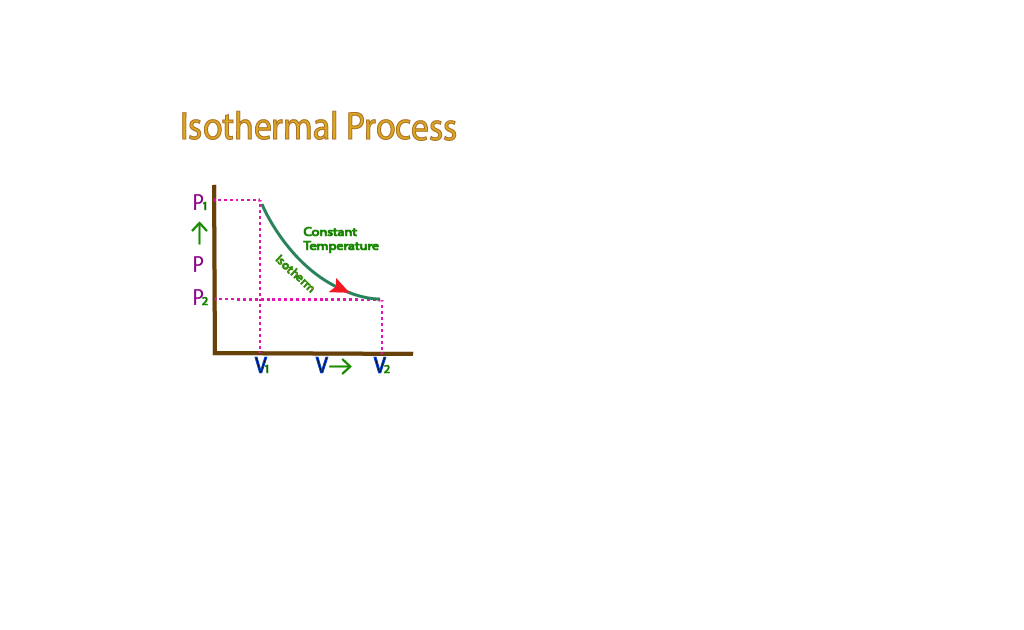
The transfer of heat from one place to another requires time, so to keep the temperature of the gas constant, the expansion or compression must take slowly.
Types of isothermal process
There are two types of isothermal processes.
1. Constant Temperature Process
2. Constant Pressure Process
Constant Temperature Process
This process is used to keep the temperature of any medium in a fixed range. Here is an example of this process.
Consider that there is a thermometer kept on a container filled with water. If you want to keep the temperature constant, then the container can be filled with ice water or warm water. Now, the water in the container will start heating up and the thermometer will start showing the increasing temperature of the water. The water will stop heating up once it reaches the temperature that is desired by you. So, it is a constant temperature process.
Constant Pressure Process
This is the process of maintaining the pressure of the system under observation. Here is an example of this process.
Consider that you are taking a hot air balloon and you want to keep the temperature of the balloon at a fixed level. You can use an electric heater to keep the temperature of the hot air balloon constant. The amount of electricity that the heater uses depends on the pressure of the hot air balloon.
As the pressure of the balloon increases the heater will use more energy to keep the temperature constant. When the pressure of the balloon reduces, the heater will not work to maintain the temperature.
These are the two types of isothermal processes. The isothermal process is the process where the heat transfer rate stays almost constant while the temperature varies. So, it is the process of keeping the desired temperature constant.





Leave a Reply