Atomic Spectra | Atomic Spectroscopy, Rydberg’s Formula
Atomic spectra are defined as during transitions between different energy levels within an atom, the spectrum of the electromagnetic waves emitted or absorbed by an electron is revealed.
What is Atomic Spectra?
The spectrum of frequencies of radiation emitted or absorbed during transitions of electrons between Energy levels within an atom is known as the Atomic spectra. The elements have a characteristic Spectrum through which they can easily be recognized. In an atom, electrons have a certain amount of energy.
There are more Energy states in a tom than there are electron states. When an electron moves from one Energy level to another, it emits light or photon with a specific wavelength. When an electron is excited from one Energy level to another, it emits or absorbs light from a specific wavelength.
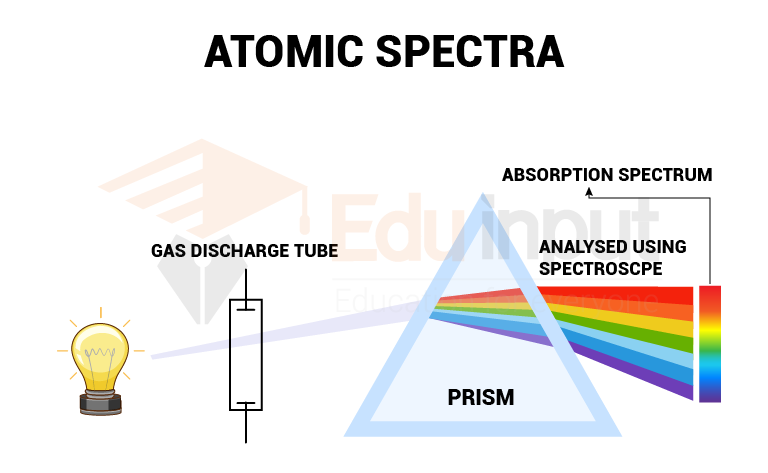
Atomic Spectroscopy
The study of the absorption or emission of radiation by atoms is known as atomic spectroscopy.
Atomic Spectroscopy has three types
Atomic emission spectroscopy
The transfer of energy from the ground state to the excited state is called excited state transfer. An explanation of the electronic transition can be found in the atomic emission.
Atomic absorption spectroscopy:
The energy differences between the lower and higher levels should be the same for absorption to take place. The principle of atomic absorption spectroscopy is based on the fact that free electrons can absorb radiation at a specific wavelength. The absorption of ground-state atoms in the gaseous state is quantized by it.
Atomic fluorescence spectroscopy:
An analytical method used to determine the concentration of elements in samples is known as atomic fluorescence spectrometry. The element of interest is excited to a higher electronic energy level by a light source when the sample is converted to gaseous atoms.
Rydberg’s Formula
In atomic physics, the wavelength of the line in a wide range of chemical elements is determined by the equation. A generalization of the Balmer series for atomic hydrogen transitions is what the equation is about. The ground-state energy of the electron in the Bohr model of the hydrogen atom is explained in terms of a unit of energy.
The Rydberg’s Formula is:
1/λ=R(1/n′2−1/n2)
Spectral Series
A predictable pattern is followed by the light frequencies emitted by certain elements. Because hydrogen is the most basic atom, it has the most basic spectrum. The lines in the hydrogen spectrum appear to lack order or regularity, but the spacing between them decreases on a regular basis, and each of these sets is referred to as a spectral series.
The Balmer series was discovered in the visible region of the Hydrogen Spectrum, by a Swedish teacher.
Uses of Atomic Spectroscopy
In the pharmaceutical industry, it’s used to find traces of the materials used. It is useful for studying more than one element at a time. It’s useful for studying the structure of atoms and molecules.
An accurate method for finding components in a material with an unknown chemical composition can be provided by it. It can be used to identify the lines of the materials used in metallurgy. Environmental and occupational monitoring can be done with the help of atomic spectroscopy.





Leave a Reply