Boron-Discovery, Properties, And Applications
Boron is a chemical element with the symbol B and atomic number 5. It is a metalloid and is found in nature in the form of compounds. Boron has many applications in various industries such as agriculture, nuclear energy, and materials science. In this article, we will discuss the discovery, physical and chemical properties, facts, and applications of boron.
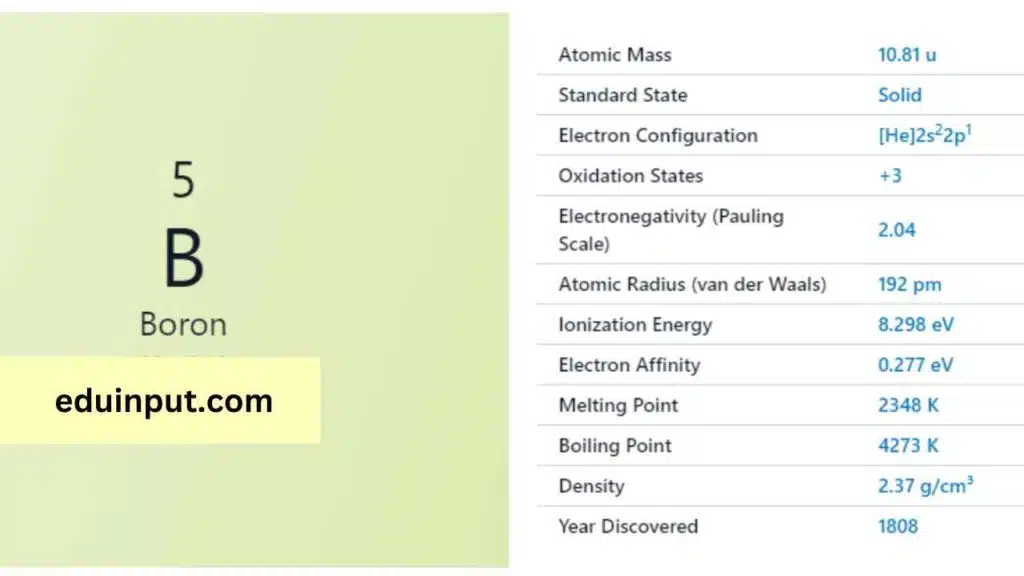
| Property | Value |
| Name | Boron |
| Symbol | B |
| Atomic number | 5 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Black |
| Classification | Semi-metallic |
| Period in the periodic table | 13 |
| Group name | (none) |
| Block in the periodic table | 2 |
| Block in periodic table | p |
| Shell structure | 2.3 |
| CAS Registry | 7440-42-8 |
Discovery
Boron was discovered by two French chemists, Joseph Louis Gay-Lussac, and Louis Jacques Thénard, in 1808. However, Sir Humphry Davy and Jöns Jakob Berzelius are also credited with the discovery of boron. It was first isolated in its pure form by the American chemist W.H. Weintraub in 1909.
Physical Properties
Boron is a hard, black, crystalline solid with a melting point of 2076°C and a boiling point of 4000°C. It has a density of 2.34 g/cm³ and is a poor conductor of electricity at room temperature. Boron is a brittle metalloid and is not ductile or malleable.
Chemical Properties
Boron is a chemical element with a unique electronic configuration that makes it reactive in certain chemical reactions. It is a non-metal and has three valence electrons in its outer shell.
Boron can form covalent bonds with other elements, and it is often used as a dopant in semiconductor devices. Boron is also known to form complex ions with other elements, such as the borate ion, which is found in various minerals.
Facts
- Boron is the only non-metal in group 13 of the periodic table.
- It is the fifth most abundant element in the Earth’s crust.
- Boron has two stable isotopes: boron-10 and boron-11.
- Boron is an essential element for plant growth, and it is often added to soil as a fertilizer.
Applications
Boron has a wide range of applications in various industries. Some of the major applications of boron are:
- Agriculture: Boron is used as a fertilizer to improve crop yields and prevent plant diseases.
- Nuclear energy: Boron is used as a neutron absorber in nuclear reactors to control the rate of fission reactions.
- Materials science: Boron is used in the manufacture of ceramics, glass, and high-strength alloys.
- Semiconductor industry: Boron is used as a dopant in the manufacture of semiconductor devices, such as diodes and transistors.
- Health: Boron supplements are often used to treat arthritis and osteoporosis.
Boron is a versatile chemical element that has numerous applications in various industries. Its unique physical and chemical properties make it a valuable material for manufacturing processes and scientific research.







Leave a Reply