Difference Between Endothermic And Exothermic Reactions
October 11, 2023
The main difference between endothermic and exothermic reactions is the direction of energy transfer, which is opposite.
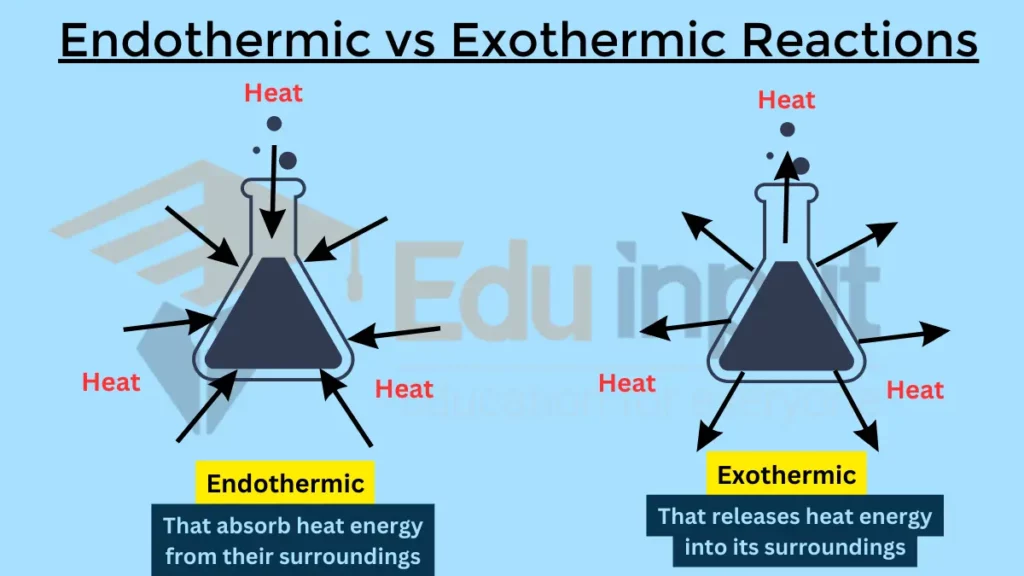
Endothermic vs Exothermic Reactions
Here are the main Difference Between Endothermic And Exothermic Reactions:
| Characteristic | Endothermic reaction | Exothermic reaction |
|---|---|---|
| Energy transfer | Absorbs energy from surroundings | Releases energy to surroundings |
| ΔH | Positive | Negative |
| Temperature | Decreases | Increases |
| Enthalpy of products | Higher than enthalpy of reactants | Lower than enthalpy of reactants |
| Entropy change | Increases or decreases | Increases |
| Spontaneity | Spontaneous at high temperatures | Spontaneous at all temperatures |
| Examples | Melting ice, photosynthesis | Burning wood, respiration |
| Applications | Refrigeration, air conditioning, food processing | Power generation, heating, transportation |
| Effect on surroundings | Cools surroundings | Heats surroundings |
| Safety | Generally safe | Can be dangerous if not handled properly |
| Reversibility | Usually reversible | Usually reversible |
| Environmental impact | Can be beneficial or harmful depending on the reaction | Can be beneficial or harmful depending on the reaction |
Also Read;
| What is Thermochemistry? | Spontenous Reactions |
| Non-Spontenous Reactions | Difference between Spontaneous and Non-Spontaneous Reactions |
File Under:



Leave a Reply