15 Examples of Substitution Reactions
Substitution reactions are fundamental processes in chemistry where one atom or group of atoms in a molecule is replaced by another atom or group. These reactions are crucial in organic and inorganic chemistry, playing a significant role in the synthesis of various compounds. In this article, we’ll explore 15 very common examples of substitution reactions.
Examples of Substitution Reactions
Here are 15 Examples of Substitution Reactions:
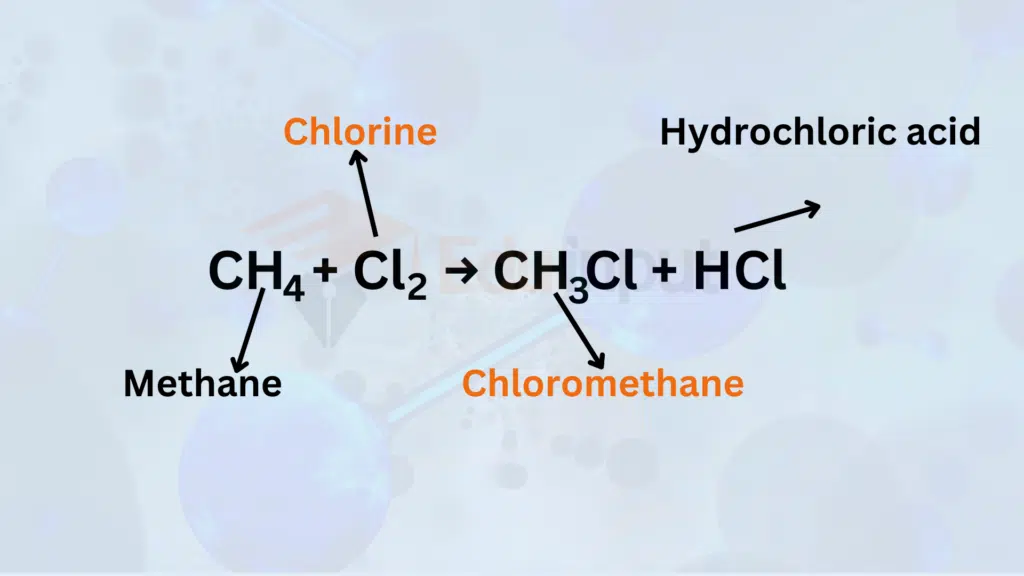
1. Alkane Halogenation – Chlorination of Methane
In alkane halogenation, substitution reactions occur. For example, the chlorination of methane involves the replacement of a hydrogen atom with a chlorine atom, forming chloromethane and hydrochloric acid.
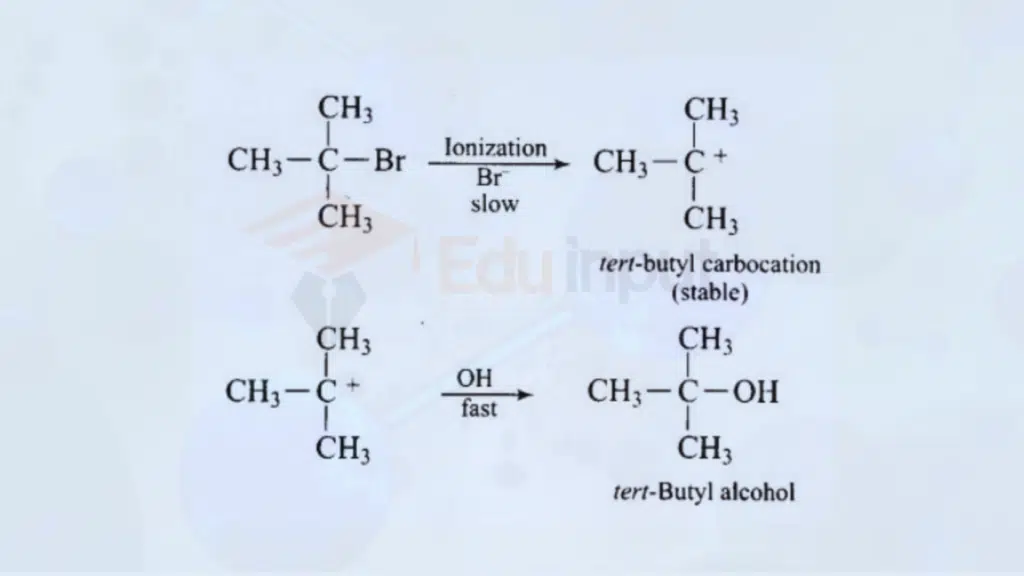
2. Nucleophilic Substitution – SN1 Reaction
Nucleophilic substitution is a common organic reaction. In the SN1 reaction, a leaving group is replaced by a nucleophile. An example is the hydrolysis of tertiary alkyl halides.
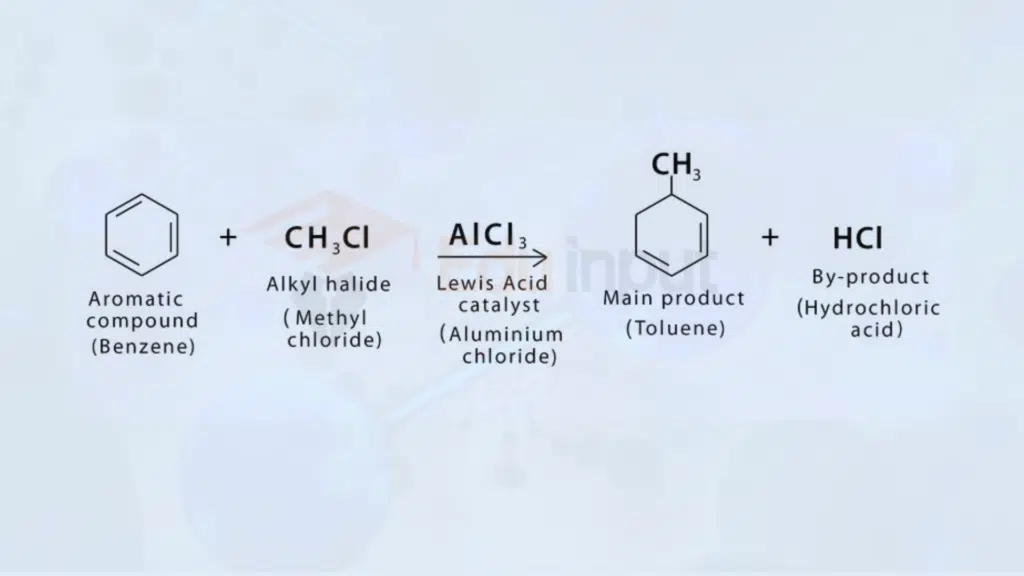
3. Aromatic Substitution – Friedel-Crafts Reaction
In aromatic substitution, the Friedel-Crafts reaction is noteworthy. It involves the substitution of a hydrogen atom on an aromatic ring with an alkyl or acyl group.
4. Electrophilic Substitution – Bromination of Benzene
Electrophilic substitution occurs in the bromination of benzene, where a hydrogen atom is replaced by a bromine atom, resulting in the formation of bromobenzene.

5. Biological Substitution – Enzymatic Reactions
Biological systems involve substitution reactions. In enzymatic reactions, a functional group in a biomolecule is often replaced or modified, playing a crucial role in various metabolic pathways.
6. Organometallic Substitution – Grignard Reaction
Organometallic compounds participate in substitution reactions. In the Grignard reaction, an alkyl or aryl magnesium halide reacts with an organic halide, leading to the substitution of the halide group.
7. Solvolysis – Alcoholysis of Esters
Solvolysis reactions involve the substitution of a leaving group with a solvent molecule. For instance, alcoholysis of esters results in the formation of alcohols and carboxylic acids.
8. Halide Substitution in Coordination Compounds
Coordination compounds undergo substitution reactions. Halide ligands in a complex may be replaced by other ligands, impacting the compound’s properties.
9. Sulfonation in Aromatic Compounds
Aromatic compounds can undergo substitution reactions such as sulfonation. Here, a hydrogen atom on the aromatic ring is replaced by a sulfonic acid group.
10. Radical Substitution – Chlorination of Alkanes
Radical substitution reactions involve the replacement of hydrogen atoms in alkanes. The chlorination of alkanes in the presence of ultraviolet light is an example.
11. Oxidative Substitution in Transition Metals
Transition metal complexes can undergo oxidative substitution reactions. Ligands are replaced through oxidation processes, altering the coordination sphere.
12. Acylation in Organic Synthesis
Acylation involves the substitution of an acyl group into a molecule. This reaction is crucial in organic synthesis, especially in the formation of amides.
13. Ammonolysis – Substitution in Amides
Amides can undergo ammonolysis, a substitution reaction where an amide is treated with ammonia or an amine, leading to the replacement of the carbonyl oxygen.
14. Nucleophilic Aliphatic Substitution – SN2 Reaction
In the SN2 reaction, a nucleophile attacks the substrate, resulting in the simultaneous expulsion of a leaving group. This process is common in aliphatic compounds.
15. Tautomerization – Keto-Enol Tautomerism
Tautomerization involves the rapid interconversion of isomeric forms. In keto-enol tautomerism, a hydrogen atom is substituted by a double bond, leading to structural isomers.




Leave a Reply