10 Examples of Wave Particle Duality
Wave particle duality is a fundamental concept in quantum mechanics that describes how particles, such as electrons and photons, exhibit both wave-like and particle-like properties. Examples of wave particle duality include electron diffraction and Photoelectric Effect.
Examples of Wave Particle Duality
Here are ten examples of wave-particle duality in action.
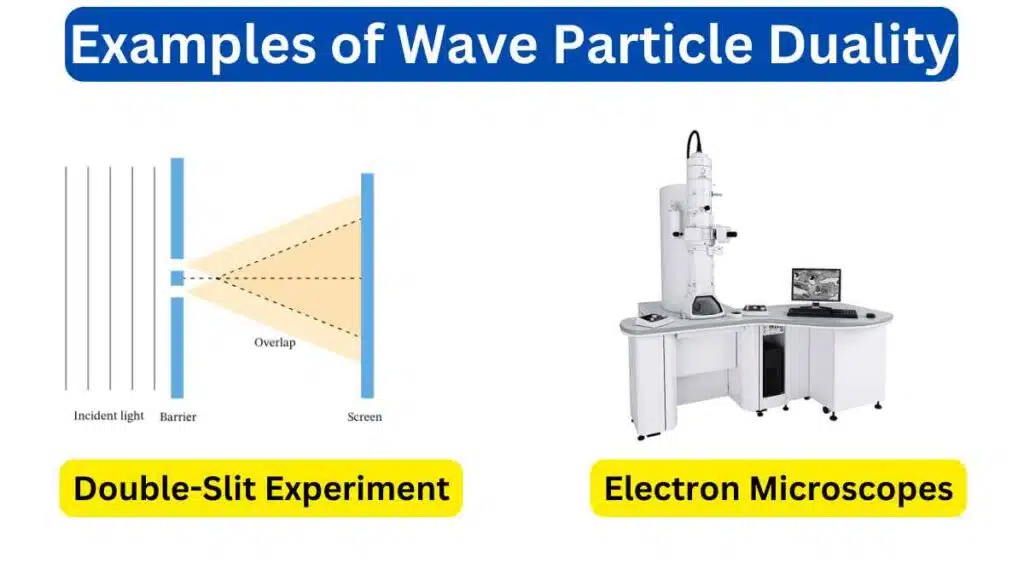
1. Double-Slit Experiment
In the double-slit experiment particles like electrons or photons are sent through two slits in a barrier. When unobserved, they create an interference pattern on a screen, behaving like waves. However, when observed, they behave as particles, producing two distinct bands on the screen.
2. Electron Diffraction
Electron diffraction experiments use beams of electrons to create diffraction patterns when they encounter a crystalline lattice. These patterns resemble those produced by waves, demonstrating the wave-like nature of electrons.
3. Photoelectric Effect
The photoelectric effect involves the emission of electrons from a material when it is exposed to light. This phenomenon is explained by the particle-like behavior of photons, which transfer their energy to electrons, causing them to be ejected from the material.
4. Compton Scattering
Compton scattering occurs when X-rays collide with electrons. The scattered X-rays change their wavelength, exhibiting both wave-like interference and particle-like momentum transfer, confirming the duality of particles and waves.
5. Davisson-Germer Experiment
The Davisson-Germer experiment demonstrated that electrons, when fired at a crystal, produce diffraction patterns similar to X-rays. This provided further evidence of the wave-particle duality of electrons.
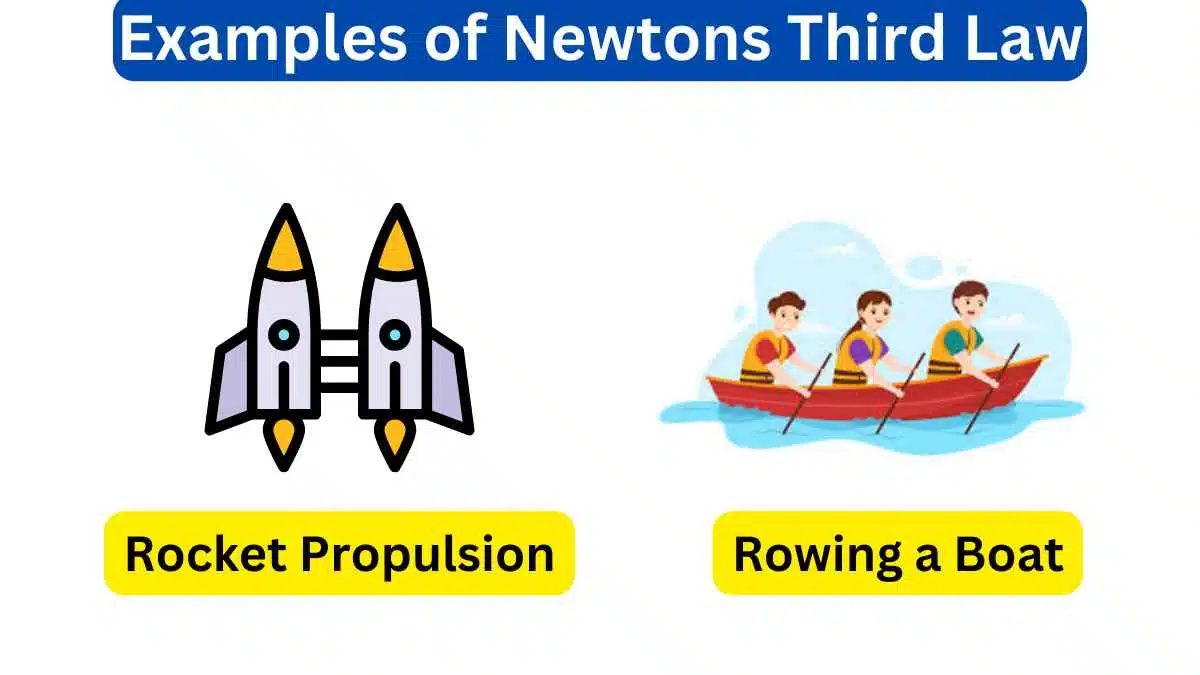
6. Electron Microscopes
Electron microscopes use the wave-like behavior of electrons to achieve high-resolution imaging. Electron beams exhibit interference patterns that help magnify and visualize tiny structures, showcasing both their wave and particle attributes.
7. Wavepackets
Wavepackets are localized wave-like solutions to the Schrödinger equation in quantum mechanics. They describe how particles like electrons are distributed in space and exhibit characteristics of both waves and particles.
8. De Broglie Wavelength
Louis de Broglie proposed that all particles, not just light, exhibit wave-like properties. The de Broglie wavelength relates the momentum of a particle to its wavelength, highlighting the duality of matter.
9. Matter Waves
Matter waves describe how particles, such as electrons, can be associated with a wave function that represents their probability distribution in space. These wavefunctions exhibit interference patterns, emphasizing their wave-like nature.
10. Electron Tunneling
In tunneling phenomena, electrons can pass through energy barriers that classical physics would consider impenetrable. This behavior is explained by the wave-like nature of electrons, allowing them to “tunnel” through the barrier.
These examples illustrate the wave-particle duality of particles in the realm of quantum mechanics, where the behavior of particles cannot be solely described as either waves or particles but involves a combination of both aspects depending on the experimental context.




Leave a Reply