10 Examples of Gamma Decay
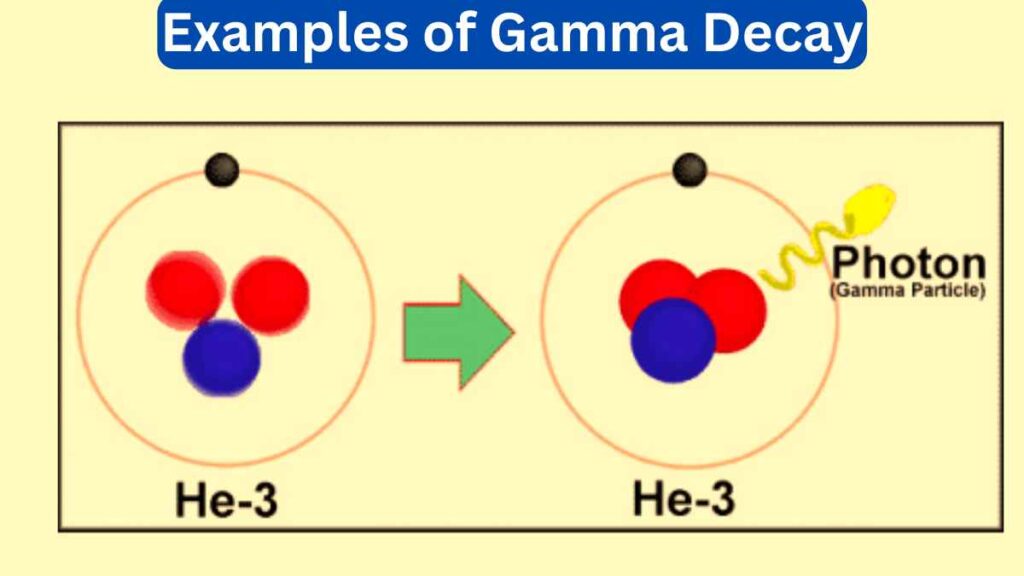
Gamma decay also known as gamma emission is a nuclear process in which an unstable atomic nucleus releases a high-energy gamma-ray photon to transition to a more stable state. Gamma rays are the most energetic form of electromagnetic radiation and gamma decay plays a significant role in nuclear physics, astrophysics, and medical imaging.
Examples of Gamma Decay
These ten examples of gamma decay highlight the applications and consequences of this nuclear process.
1. Cobalt-60 Decay
Cobalt-60 (Co-60) is a radioactive isotope used in medicine and industry. In its decay, a neutron in the nucleus is transformed into a proton, releasing a high-energy gamma-ray photon in the process. This gamma radiation is utilized in radiation therapy for cancer treatment.
2. Technetium-99m Decay
Technetium-99m (Tc-99m) is widely used in medical imaging for procedures like single-photon emission computed tomography (SPECT). During its decay, it emits gamma rays that are detected by imaging devices, allowing for the visualization of internal structures in the human body.
3. Sodium-24 Decay
Sodium-24 (Na-24) is used in research and industrial applications. It decays by emitting a high-energy gamma-ray photon, contributing to the production of radioactive tracers and gamma-ray sources.
4. Iron-59 Decay
Iron-59 (Fe-59) is a radioactive isotope used in nuclear medicine. It undergoes gamma decay as it transitions to a more stable state, releasing gamma radiation used in radiopharmaceuticals.
5. Phosphorus-32 Decay
Phosphorus-32 (P-32) is utilized in biological and medical research. During its decay, it emits gamma rays that are employed in radiation therapy and as tracers for studying metabolic processes in living organisms.
6. Iodine-131 Decay
Iodine-131 (I-131) is widely used in nuclear medicine to treat thyroid conditions and diagnose diseases. Its decay includes the emission of gamma rays that can be detected to image the thyroid gland.
7. Cesium-137 Decay
Cesium-137 (Cs-137) is a radioactive isotope formed in nuclear reactors and nuclear weapons tests. Its gamma decay is a significant source of radiation in contaminated areas, and it is a subject of concern in nuclear fallout.
8. Carbon-14 Decay
Carbon-14 (C-14) is used in radiocarbon dating to determine the age of ancient artifacts and fossils. During its decay, it emits beta particles and gamma rays, allowing for precise dating of materials.
9. Potassium-40 Decay
Potassium-40 (K-40) is a naturally occurring radioactive isotope found in potassium-containing minerals. It undergoes beta decay followed by gamma decay, contributing to natural background radiation.
10. Uranium-235 Decay
Uranium-235 (U-235) is a fissile isotope used in nuclear reactors and weapons. Its gamma decay occurs as part of the overall decay process, releasing high-energy gamma rays in the nuclear fuel.

 written by
written by 



Leave a Reply