Gold-Discovery, Properties, And Applications
Gold is a chemical element with the symbol Au and atomic number 79. It is a highly sought-after precious metal that has been used for various purposes throughout history, including currency, jewelry, and decoration. It is also used in a variety of modern applications, such as electronics, dentistry, and medicine.
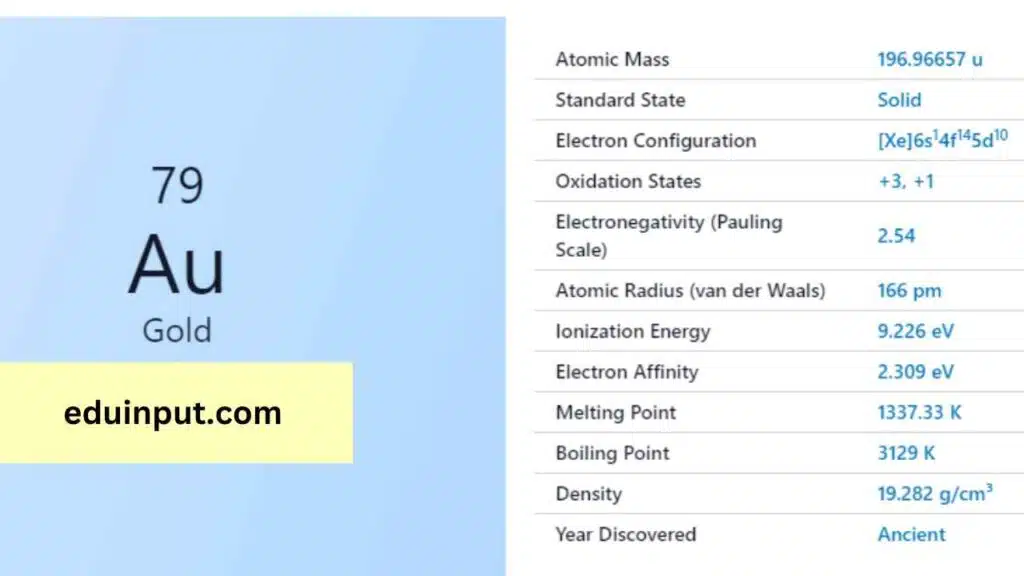
| Property | Value |
| Name | Gold |
| Symbol | Au |
| Atomic number | 79 |
| Relative atomic mass (Ar) | 196.966569 |
| Standard state | Solid at 298 K |
| Appearance | Gold |
| Classification | Metallic |
| Group in periodic table | 11 |
| Group name | Coinage metal |
| Period in periodic table | 6 |
| Group in the periodic table | d |
| Shell structure | 2.8.18.32.18.1 |
| CAS Registry | 7440-57-5 |
Discovery
Gold has been known since ancient times and was highly valued by many cultures. The first recorded discovery of gold in modern times occurred in the late 17th century by Sir Isaac Newton. Gold was later discovered in various parts of the world, including California, South Africa, and Australia.
Physical Properties
Gold is a soft, yellow metal that is ductile and malleable. It has a melting point of 1,064 degrees Celsius and a boiling point of 2,700 degrees Celsius. Gold is a good conductor of electricity and does not corrode or tarnish, making it an ideal material for electrical contacts and jewelry.
Chemical Properties
Gold is a highly unreactive element and does not form compounds easily. It is generally found in its pure form in nature and is often extracted from ores using chemical processes such as cyanide leaching. Gold has a unique ability to resist oxidation and is therefore used in a variety of applications where resistance to corrosion is important.
Facts
- Gold is so rare that all of the gold ever mined could fit into a cube that is 21 meters on each side.
- The term “carat” is used to describe the purity of gold. Pure gold is 24 carats, while 18-carat gold is 75% gold and 25% other metals.
- Gold has been used as currency for thousands of years and was the basis of the global monetary system until the 20th century.
Applications
- Jewelry: Gold is a popular choice for jewelry due to its beauty, rarity, and resistance to corrosion.
- Electronics: Gold is an excellent conductor of electricity and is used in a variety of electronic applications, including smartphones and computers.
- Medicine: Gold has several medical applications, including the treatment of rheumatoid arthritis and some forms of cancer.
- Dentistry: Gold is used in dental fillings and crowns due to its biocompatibility and resistance to corrosion.




Leave a Reply