Hess’s Law-Definition, Application, And Examples
Definition of Hess’s Law
Hess’s Law states that the overall enthalpy change of a reaction is equal to the sum of the enthalpy changes of the individual steps in the reaction, regardless of the pathway taken.
Mathematical definition of Hess’s Law
ΔH°reaction = ΣΔH°f(products) - ΣΔH°f(reactants)
where:
- ΔH°reaction is the overall enthalpy change of the reaction
- ΣΔH°f(products) is the sum of the standard enthalpies of formation of the products
- ΣΔH°f(reactants) is the sum of the standard enthalpies of formation of the reactants
Explanation
Hess’s law of constant heat summation states that the overall enthalpy change for a chemical reaction is the sum of the enthalpy changes of the individual steps of the reaction, regardless of the pathway taken.
This law is a consequence of the fact that enthalpy is a state function, meaning that its value depends only on the initial and final states of the system, and not on the path taken between those states.
Hess’s law can be used to calculate the enthalpy change of a reaction even if it cannot be measured directly.
For example, the enthalpy of formation of carbon monoxide (CO) cannot be measured directly because CO is unstable and tends to be disproportionate into carbon and carbon dioxide. However, we can use Hess’s law to calculate the enthalpyof thee formation of CO by considering the following cycle:
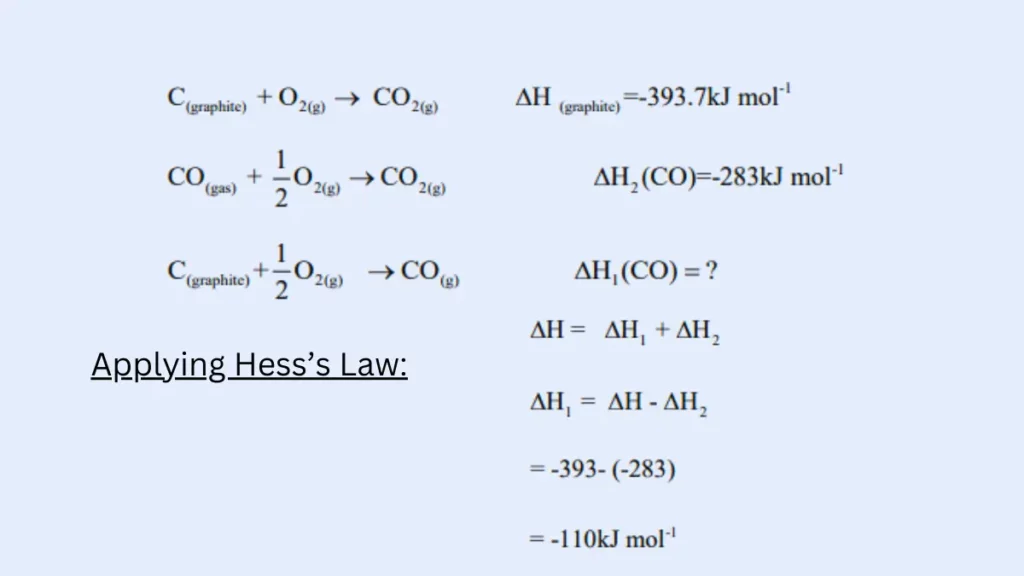
Verification of Hess’s law
The enthalpy of formation of sodium carbonate cannot be measured directly because Na2CO3 is a solid and CO2 and O2 are gases. However, we can use Hess’s law to calculate the enthalpy of formation of Na2CO3 by considering the following cycle.
According to Hess’s law, the enthalpy change of the overall reaction will be the same, regardless of which pathway is taken. This is because the enthalpy change is a state function, meaning that it depends only on the initial and final states of the system, not on the pathway taken between those states.
If we start at state A and end at state D, the overall enthalpy change will be the same, regardless of whether we take a direct path from A to D or a more indirect path through states B and C.
The formation of sodium carbonate is a good example of this. The formation of sodium carbonate can be written as both a single-step and two-step reaction:
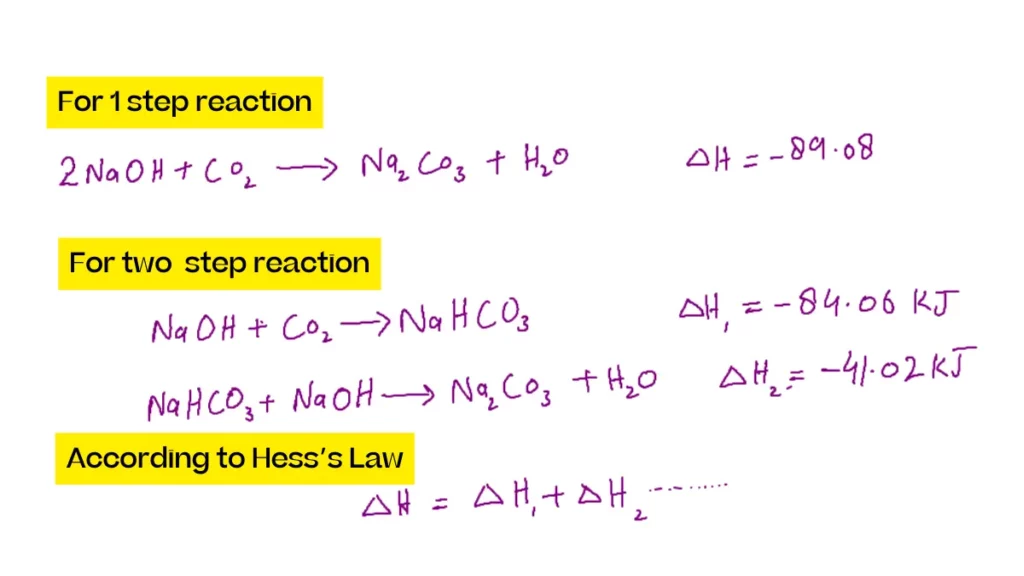
Example
Here is an example of how to use Hess’s Law:
Desired reaction
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
Enthalpy of combustion of methane
CH4(g) + 2O2 (g) → CO2(g) + 2H2O(g) ΔH = -890.4 kJ/mol
Enthalpy of formation of water
H2(g) + 1/2O2(g) → H2O(g) ΔH = -285.8 kJ/mol
Hess’s Law calculation:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) ΔH = -890.4 kJ/mol
2H2(g) + 1/2O2(g) → H2O(g)) ΔH = -2(285.8 kJ/mol) = -571.6 kJ/mol
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) ΔH = -890.4 kJ/mol – 571.6 kJ/mol = -1462 kJ/mol
Therefore, the enthalpy change of the desired reaction is -1462 kJ/mol.
Hess’s Law Cycle
Here is how you can find enthalpy change by making Hess’s Law cycle of reactants, products and their enthalpies:
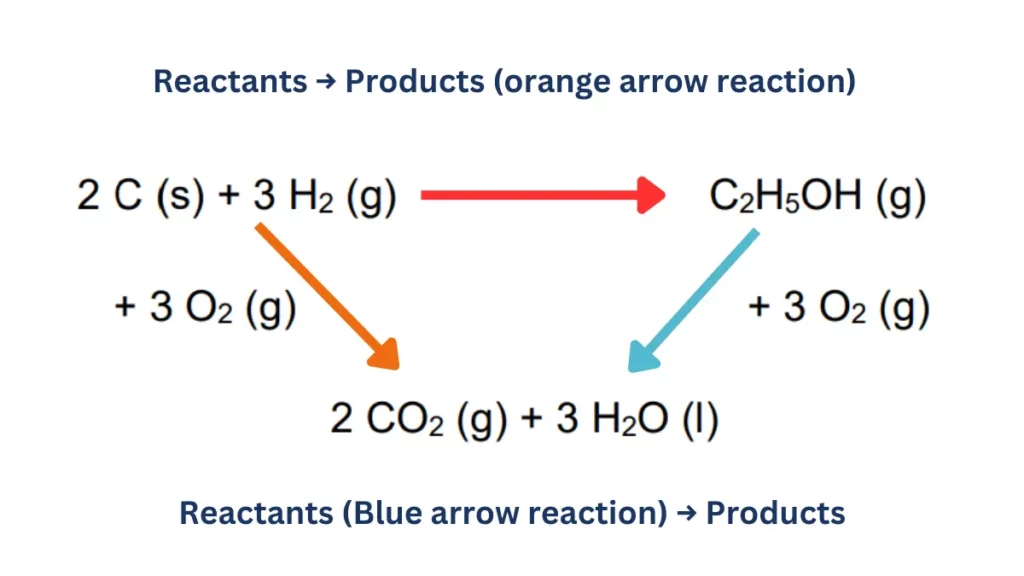
Also Read:
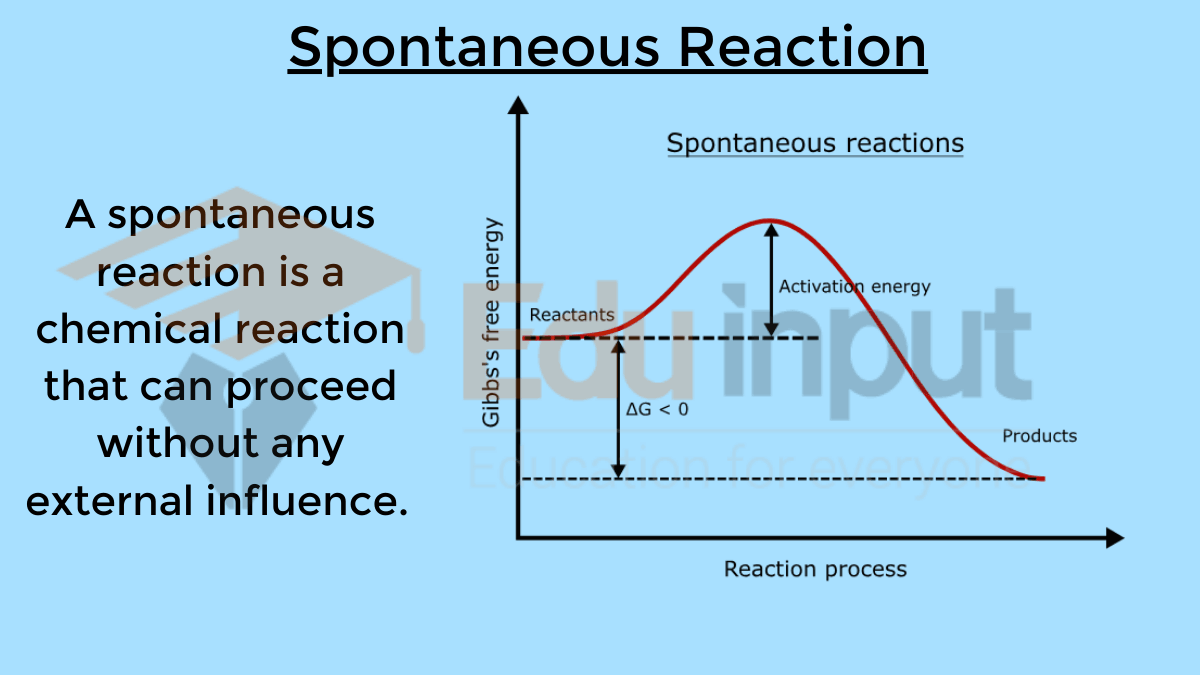
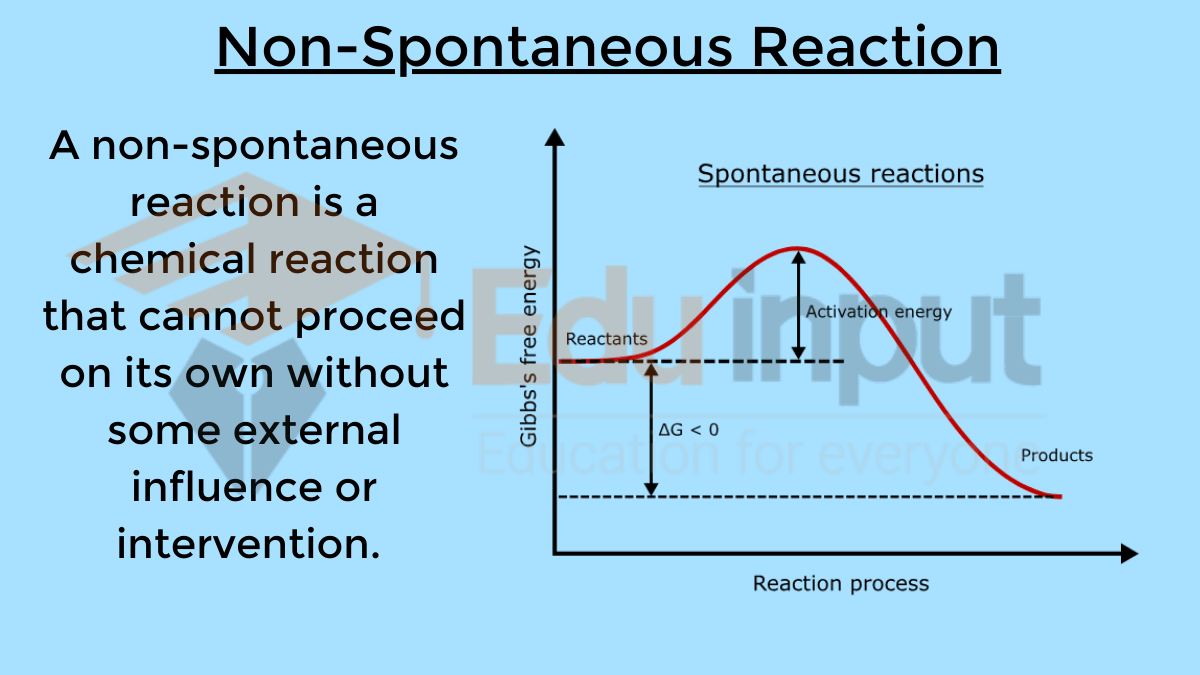
| Thermochemistry | Non-Spontaneous Reactions | Spontaneous Reactions | |
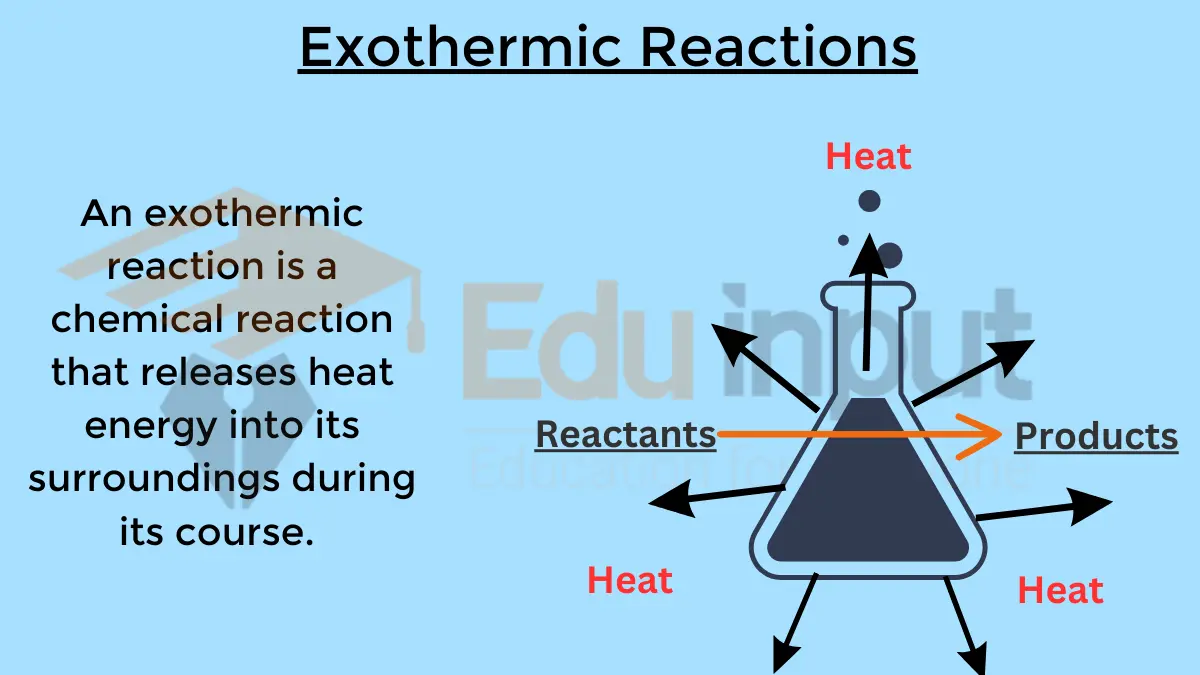
| Endothermic Reactions | Exothermic Reactions |



Leave a Reply