Magnetic Properties of Solids | Domain Theory of Ferromagnetism
To understand the origin of the magnetic properties of solids, the magnetic field produced by bar magnets and the electric current must be understood.
Magnetic Properties of Solids
It is a common experience that the magnetic field produced by a long bar magnet is the same as that produced by the long solenoid-carrying current.
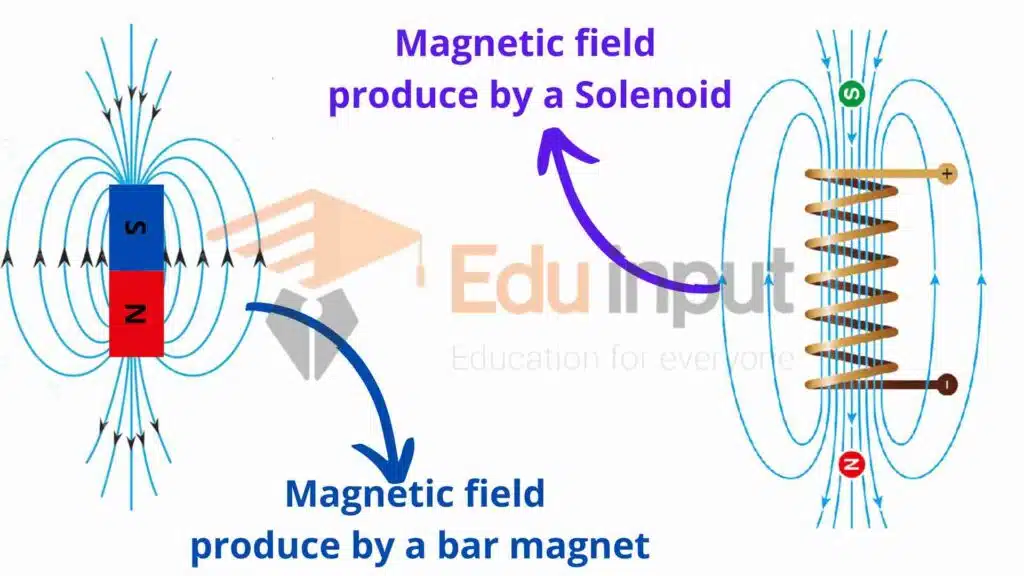
Similarly, the magnetic field produced by a small bar magnet is the same as that produced by a single loop.
Ampere thought that the magnetic effects may be due to the circulating currents i.e. moving electric charges. But at his time the structure of the atom was unknown.
Since now the structure of the atom is known, one can imagine the orbiting electron to be a circulating current.
Magnetic Field due to Spin and Orbital Motion
There are two types of electronic motions
- Due to spin
- Due to orbital motion
An electron in orbital motion is equivalent to the current loop on atomic dimensions. Due to this current, there is a magnetic field just like the magnetic field produced due to a single loop.
Due to spin, there is another magnetic field called the spin magnetic field. The net magnetic field in an atom is due to the combined effect of the magnetic fields produced by spin and orbital motion.
Since there is a number of electrons in an atom, so their orientations may also be in different directions. Due to these different directions the magnetic fields may cancel each other or may strengthen each other.
An atom in which there is a net magnetic field acts like a tiny magnet and is called a magnetic dipole.
The magnetic fields of the atoms are responsible for the magnetic behavior of the substances made up of these atoms. Magnetism is therefore due to the orbital and spin motion of the electrons orbiting around the nucleus and is thus the property of all substances.
Nuclear Spin
The nucleus itself spins about its axis but due to the nuclear spin, the magnetic produced is so small that it is not taken into account. Thus the source of magnetism of an atom is the electrons.
No Monopoles
It is now clear that no attempt can be made to obtain a single magnetic pole. Because a single electronic current loop behaves like a magnet so it is not possible to separate one pole from the other. It is an experimental reality.
Magnetic Substances
Depending upon the net magnetic effect in atoms the substances are classified as under.
Paramagnetic Substances
In these substances, the orbits and the spin axes of electrons in an atom are so oriented that their fields support each other and the atom behaves like a tiny magnet. Substances with such atoms are called paramagnetic substances.
Diamagnetic Substances
In these substances, the orbits and the spin axes of the electrons in an atom are so oriented that their fields cancel each other and the net magnetic field is zero. These are called diamagnetic substances. Water, copper, bismuth, and antimony are diamagnetic substances.
Ferromagnetic Substances
There are certain solids in which the atoms cooperate in such a way that their individual magnetic fields are added to exhibit a strong magnetic effect, such substances are called ferromagnetic/substances.
Fe, Co, Ni, Chromium dioxide, and Alnico are ferromagnetic substances.
Domain Theory of ferromagnetism
Small regions exist in ferromagnetic substances and these regions are called domains.
The domains are of the macroscopic size of millimeters or less but large enough to contain 1012 to 1016 atoms. Within each domain, the magnetic field of all spinning electrons is parallel to one another i.e. each domain is magnetized to saturation.
Each domain behaves like a small magnet with its own south and north poles. In the absence of an external magnetic field, the domains are randomly directed such that the net magnetic effects of the specimen are zero.
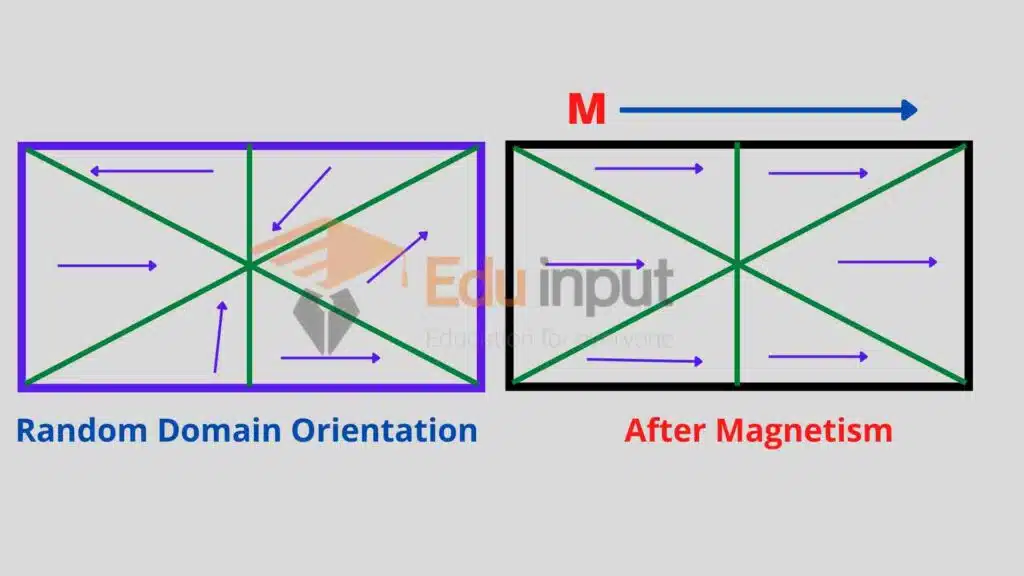
When the specimen is placed in the external magnetic field as that of a solenoid, the domains line parallel to the lines of the external magnetic field, and the entire specimen is saturated.
The specimen and the solenoid thus become a powerful magnet and are called an electromagnet.
Soft magnetic material
Iron is a soft magnetic material as its domains are easily oriented on applying an external magnetic field and also readily return to random orientations when the external magnetic field is removed. This kind of material is desired in electromagnets and transformers.
Hard magnetic material
There are also some materials that are not easily oriented to order. They require very strong external magnetic fields, but once oriented retain the alignment. They are called hard magnetic materials and steel is one of them.
Another such material is Alnico V.
Curie temperature
The thermal vibrations tend to disturb the orderliness of the domains. Ferromagnetic materials retain the order of their domains at ordinary temperatures.
When these materials are heated they start to lose their properties due to thermal motion. This process continues at a certain temperature (different for different materials) called the Curie temperature.
Above the Curie, temperature iron is paramagnetic but not ferromagnetic. The Curie temperature of iron is about 750°C.







Leave a Reply