Relative Density-Factors and Calculation of Relative Density
Relative density also known as specific gravity is a fundamental physical property of materials. It is a dimensionless quantity that compares the density of a substance to the density of a reference substance typically water for liquids and solids and air or hydrogen for gases. Relative density plays an important role in various scientific, industrial, and engineering applications.
What is Relative Density?
Density is defined as the mass of a substance per unit volume. The SI unit for density is kilogram per cubic meter (kg/m³). Each substance has its unique density under specific conditions. It is considered a characteristic property of that substance.
For example, gold has a density of 19300 kg/m³, while water has a density of 1000 kg/m³. The density of a sample of a substance can be used to determine its purity and it is often compared to the density of water for ease of expression.
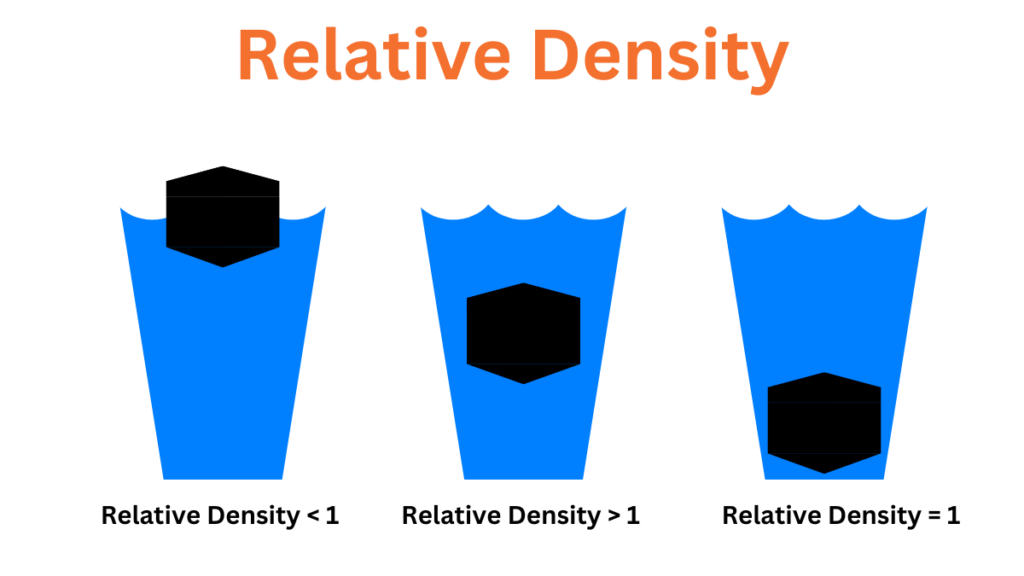
Relative density differs from density in that it is calculated relative to the density of water at room temperature and pressure which is considered the standard. The density of water is 1 gram per cubic centimeter. Specific gravity is the ratio of the mass of a substance to the mass of an equivalent volume of water. The formula to calculate specific gravity is as follows:
Specific gravity = Density of substance / Density of water
Specific gravity is a unitless quantity because it represents the ratio of similar quantities.
The specific gravity of a substance provides information about its relative mass or density and helps determine whether an object will float or sink. If the specific gravity of a substance is less than 1 it will float. If the specific gravity is greater than 1 indicates that the substance will sink.
Factors Affecting Relative Density
Several factors can influence the relative density of a substance. Understanding these factors is essential for accurately determining and interpreting relative density values. Here are some key factors that impact relative density:
- Composition: The chemical composition of a substance significantly affects its relative density. Elements and compounds have different atomic and molecular masses, which influence their densities. For example, metals tend to have higher relative densities compared to nonmetals.
- Temperature: Temperature plays a crucial role in determining the relative density of a substance, particularly for gases. As temperature increases, gas molecules gain kinetic energy and move faster, resulting in an expansion of the gas volume. This expansion decreases the gas density and, consequently, its relative density.
- Pressure: Pressure can affect the relative density of gases, especially at high pressures. Under increased pressure, gas molecules become more closely packed, leading to a higher density and relative density.
- Porosity: In the case of porous materials, such as rocks and soils, the presence of void spaces influences their relative density. Higher porosity leads to lower relative density values due to the increased volume of air or water-filled voids.
Different Ways to Calculate Relative Density
Calculating relative density involves comparing the density of a substance to the density of a reference substance. Several methods are commonly employed to determine relative density, depending on the nature of the substance. Here are three commonly used approaches:
- Direct Measurement: For solid materials, relative density can be calculated by directly measuring their mass and volume. The mass is typically determined using a balance while the volume can be obtained through various techniques like displacement or geometric measurements. The relative density is then computed by dividing the density of the substance by the density of the reference material.
- Hydrometer Method: The hydrometer method is widely used to determine the relative density of liquids. It involves using a hydrometer, which is a specialized instrument that measures the density or specific gravity of a liquid. The hydrometer floats in the liquid, and the relative density is read from a scale marked on the hydrometer.
- Gas Comparison Method: In the case of gases, the gas comparison method is commonly employed. This method involves comparing the density of the gas to the density of a reference gas, such as air or hydrogen. This can be achieved using specialized equipment like gas analyzers or by utilizing mathematical relationships, such as the ideal gas law, which relates the density of a gas to its temperature, pressure, and molar mass.
Related FAQs
What is the relative density?
Relative density, also known as specific gravity, is the ratio of the density of a substance to the density of a reference substance (usually water) at a specific temperature.
What does relative density tell us?
Relative density provides information about the heaviness or lightness of a substance compared to the reference substance.
It indicates whether a material will float or sink in the reference substance, with values greater than 1 indicating the material is denser than the reference substance and will sink, and values less than 1 indicating the material is less dense and will float.
What is mean density and relative density?
Mean density refers to the average density of a substance or material, while relative density is the ratio of the density of a substance to the density of a reference substance. Relative density is a dimensionless quantity, whereas mean density is expressed in units such as kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³).

 written by
written by 




Leave a Reply