Samarium-Discovery, Properties, And Applications
Samarium is a chemical element with the symbol Sm and atomic number 62. It is a rare earth metal that belongs to the lanthanide series. Samarium is a silvery-white metal that is malleable and ductile.
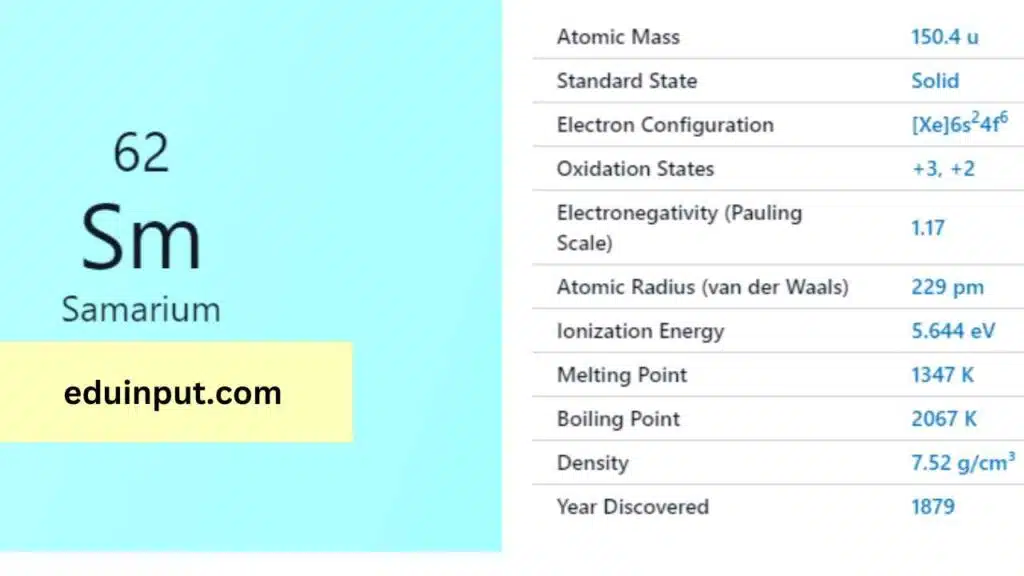
| Property | Value |
| Name | Samarium |
| Symbol | Sm |
| Atomic number | 62 |
| Relative atomic mass (Ar) | Block in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery white |
| Classification | Metallic |
| Group in periodic table | |
| Group name | Lanthanoid |
| Group in the periodic table | 6 (lanthanoid) |
| Period in the periodic table | f |
| Shell structure | 2.8.18.24.8.2 |
| CAS Registry | 7440-19-9 |
Discovery
Samarium was discovered in 1879 by French chemist Paul Émile Lecoq de Boisbaudran, who separated it from other rare earth elements.
Physical Properties
Samarium has a melting point of 1072°C and a boiling point of 1803°C. It has a density of 7.52 g/cm³ and is a silvery-white metal that is malleable and ductile.
Chemical Properties
Samarium is a rare earth metal and reacts slowly with water, but quickly with oxygen and air. It can form compounds with most non-metals and has an oxidation state of +2 or +3. Samarium is used as a dopant in optical materials, such as glass, to absorb infrared radiation.
Facts
- Samarium is named after the mineral samarskite, which was named after Russian mine official Colonel Vasili Samarsky-Bykhovets.
- Samarium has 7 naturally occurring isotopes, with ^152Sm being the most abundant.
- Samarium is used in samarium-cobalt magnets, which are some of the most powerful magnets available.
Applications
- Samarium is used in samarium-cobalt magnets, which are used in high-performance motors, headphones, and computer hard drives.
- Samarium oxide is used in nuclear reactors as a neutron absorber.
- Samariums is used in optical materials, such as glass, to absorb infrared radiation.
- Samarium is used as a catalyst in the production of synthetic rubber.





Leave a Reply