Seaborgium-Discovery, Properties, And Applications
Seaborgium, also known as Sg, is a synthetic element with the atomic number 106. It was first synthesized in 1974 by a team of researchers led by Glenn T. Seaborg, for whom the element is named. Seaborgium is a highly unstable element that has a very short half-life and is difficult to study, but it has unique properties that make it of interest to scientists.
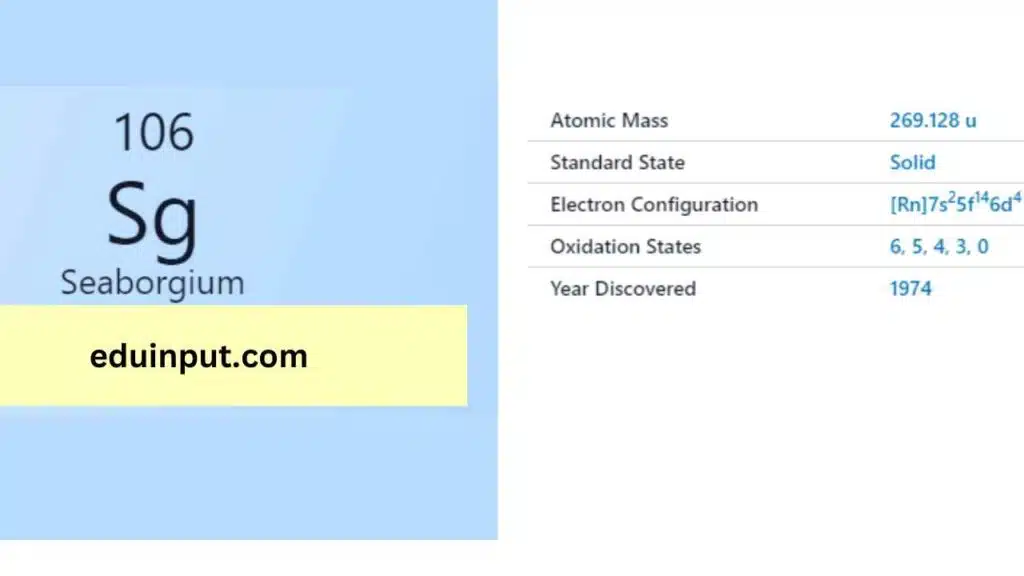
| Property | Value |
| Name | Seaborgium |
| Symbol | Sg |
| Atomic number | 106 |
| Relative atomic mass (Ar) | (longest lived isotope) |
| Standard state | Presumably a solid at 298 K |
| Appearance | Unknown, probably metallic and silvery white or grey in appearance |
| Classification | Period in the periodic table |
| Block in the periodic table | 6 |
| Group name | (none) |
| Group in the periodic table | 7 |
| (longest-lived isotope) | d |
| Shell structure | 2.8.18.32.32.12.2 |
| CAS Registry | 54038-81-2 |
Discovery
Seaborgium was first synthesized in 1974 by a team of researchers at the Lawrence Berkeley National Laboratory in California, led by Glenn T. Seaborg, for whom the element is named. The team used a heavy-ion accelerator to create seaborgium by bombarding californium-249 with oxygen-18 ions.
Physical Properties
Seaborgium is a highly unstable element that has a very short half-life, meaning it decays quickly into other elements. It is believed to have a silvery-white appearance, similar to other elements in its group. Seaborgium has an atomic weight of 269 and an estimated melting point of around 1,200°C.
Chemical Properties
Seaborgium has not been studied extensively due to its short half-life, but it is believed to have chemical properties similar to those of other elements in its group, such as tungsten and molybdenum. It is believed to have a maximum oxidation state of +6, and it may form compounds with oxygen, halogens, and other elements.
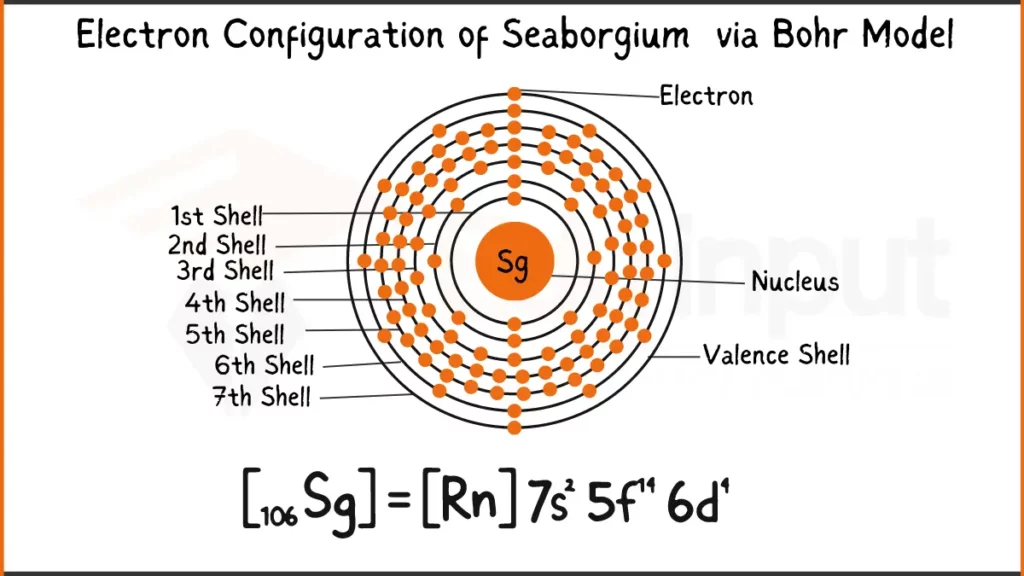
Electron Configuration of Seaborgium
Seaborgium (Sg) possesses 106 electrons. Its predicted electronic configuration is [Rn]5f¹⁴6d⁴7s², indicating it shares Radon’s (Rn) stable inner electron structure. The remaining electrons occupy the 5f subshell (14 electrons), the 6d subshell (4 electrons), and the outermost 7s subshell (2 electrons).
Electron Configuration of Seaborgium Via Bohr Model
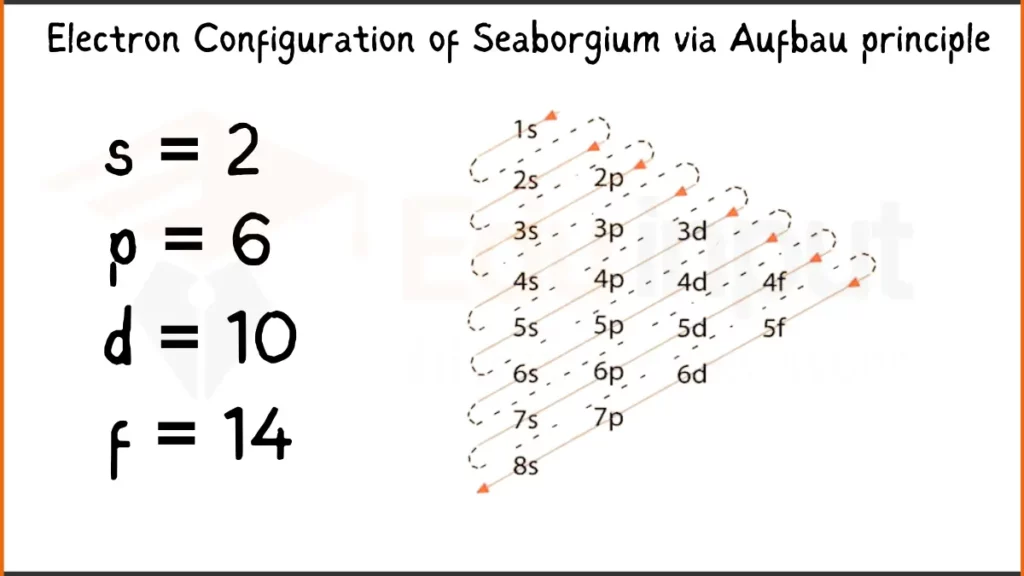
Electron Configuration of Seaborgium Via Aufbau Principle
Facts
- Seaborgium is a synthetic element that does not occur naturally on Earth.
- It is named after Glenn T. Seaborg, who was a Nobel Prize-winning chemist and one of the discoverers of several transuranium elements.
- Seaborgium is a member of the group 6 elements, which also includes chromium, molybdenum, and tungsten.
Applications
Seaborgium has no known practical applications due to its short half-life and instability. However, its discovery has helped scientists to better understand the behavior of heavy elements and the processes involved in nuclear reactions.
Seaborgium is a synthetic element that has unique properties and is of interest to scientists studying heavy elements and nuclear reactions. Although it has no known practical applications, its discovery has contributed to our understanding of the behavior of elements at the extremes of the periodic table.






Leave a Reply