Silver-Discovery, Properties, And Applications
Silver is a chemical element with the symbol Ag and atomic number 47. It is a soft, white, lustrous transition metal that exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal.
Silver has been known since ancient times, and it has a rich history of use in currency, jewelry, and other decorative objects.
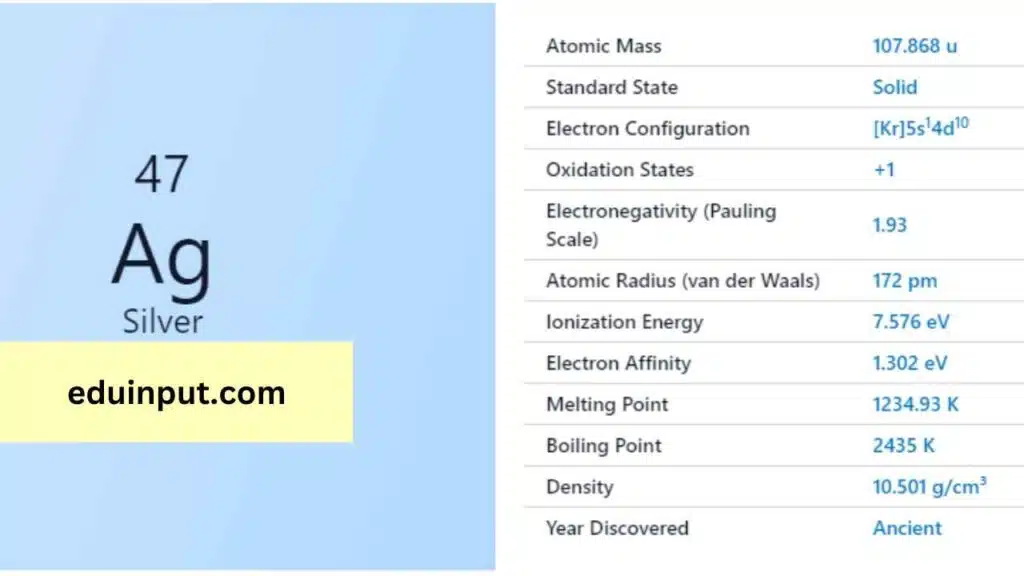
| Property | Value |
| Name | Silver |
| Symbol | Ag |
| Atomic number | 47 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silver |
| Classification | Metallic |
| Period in the periodic table | 11 |
| Group name | Coinage metal |
| Block in the periodic table | 5 |
| Block in periodic table | d |
| Shell structure | 2.8.18.18.1 |
| CAS Registry | 7440-22-4 |
Discovery
Silver has been known since ancient times and was one of the first five metals to be discovered. It was found in nugget form in the ancient city of Sumer, now Iraq, around 4,000 BC.
The ancient Egyptians mined silver in the Sinai Peninsula, and silver artifacts have been found in excavations of ancient civilizations throughout the world.
Physical Properties
Silver has a density of 10.49 g/cm³, making it one of the denser elements. It is a soft, malleable, and ductile metal with a bright, metallic luster. Silver has the highest electrical conductivity of any metal, making it an important material in electrical engineering. It is also highly reflective, making it ideal for use in mirrors and other optical devices.
Chemical Properties
Silver is a highly reactive metal and readily reacts with sulfur compounds in the air to form a black tarnish. It does not react with oxygen at room temperature, but it will burn in air if heated. Silver does not react with water, but it is soluble in nitric acid and in hot concentrated sulfuric acid.
Facts
- Silver is a precious metal that has been used as currency for thousands of years.
- The word “silver” comes from the Old English word “seolfor,” which means “silver” or “money.”
- Silver is the most reflective metal, reflecting up to 95% of visible light.
- Silver is antimicrobial and has been used in medical applications for centuries.
- The largest silver nugget ever found weighed over 2,000 pounds.
Applications
Silver is used in a wide range of applications, including:
- Currency and Jewelry
- Electronics and electrical engineering
- Mirrors and other optical devices
- Medical equipment and devices
- Photography
- Water purification
- Solar panels
Silver is a highly versatile metal with unique physical and chemical properties that make it useful in a wide range of applications. Its historical significance and ongoing demand ensure that it will remain an important material for many years to come.







Leave a Reply