Strontium-Discovery, Properties, And Applications
Strontium is a chemical element with the symbol ‘Sr’ and atomic number 38. It is a soft, silvery-white metal that is highly reactive and is found in the Earth’s crust. Strontium is known for its use in producing red fireworks, but it also has a range of other applications in various industries.
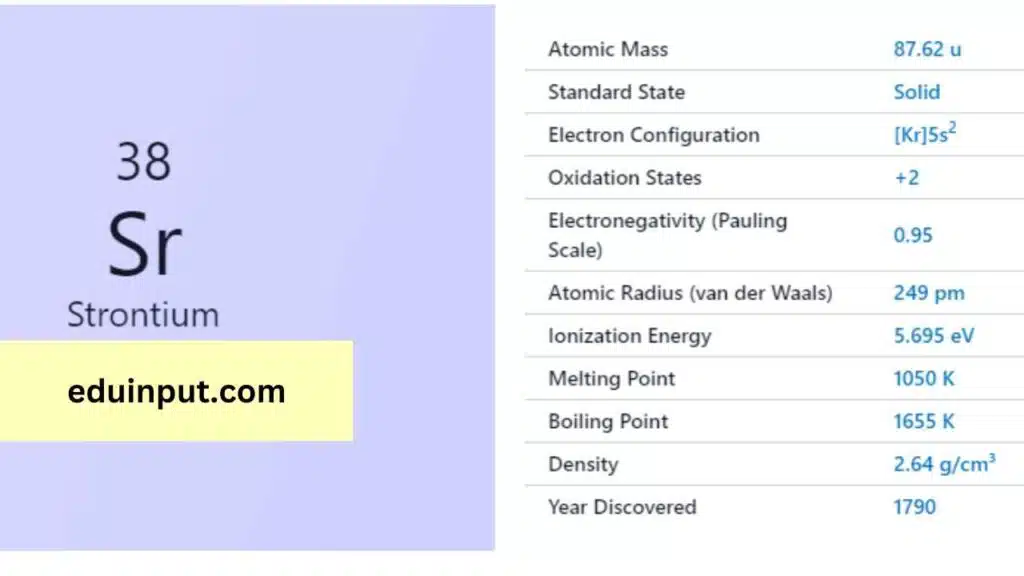
| Property | Value |
| Name | Strontium |
| Symbol | Sr |
| Atomic number | 38 |
| Relative atomic mass (Ar) | Period in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery white |
| Classification | Metallic |
| Block in the periodic table | 2 |
| Group name | Alkaline earth metal |
| Group in the periodic table | 5 |
| Block in periodic table | s |
| Shell structure | 2.8.18.8.2 |
| CAS Registry | 7440-24-6 |
Discovery
Strontium was first discovered in 1790 by Adair Crawford and William Cruickshank in Scotland. They discovered strontium in the mineral strontianite, which is named after the village of Strontian where it was found.
Physical Properties
Strontium is a soft, silvery-white metal with a density of 2.64 g/cm3. It has a melting point of 769°C and a boiling point of 1,384°C. Strontium is a highly reactive element and is easily oxidized when exposed to air.
Chemical Properties
Strontium is a highly reactive element and reacts with a variety of other elements and compounds. It reacts with water to produce strontium hydroxide and hydrogen gas. Strontium also reacts with acids to produce strontium salts.
Facts
- Strontium is named after the village of Strontian, where it was first discovered.
- Strontium has four stable isotopes and a number of radioactive isotopes.
- Strontium is used in the production of red fireworks due to its ability to produce a bright red flame.
- Strontium is also used in the production of ferrite magnets, which are used in electronic devices.
Applications
Strontium has a range of applications in various industries. Some of the major applications of strontium include:
- Pyrotechnics: Strontium is used in the production of red fireworks due to its ability to produce a bright red flame.
- Ferrite magnets: Strontium is used in the production of ferrite magnets, which are used in electronic devices such as speakers, microphones, and hard drives.
- Metallurgy: Strontium is used as a deoxidizer in the production of aluminum alloys and as a modifier in the production of cast iron.
- Medical applications: Strontium is used in some medical treatments for osteoporosis, as it can increase bone density.
Strontium is a reactive metal with diverse applications in various industries. Its ability to produce a bright red flame has made it a popular choice for use in red fireworks, while its use in ferrite magnets has revolutionized the electronics industry. Strontium’s unique physical and chemical properties make it a valuable element for various applications in metallurgy, medicine, and more.





Leave a Reply