Uncertainty Principle | Heisenberg uncertainty principle
The uncertainty principle is one of a variety of mathematical inequalities that impose fundamental limits on the accuracy of the values of certain pairs of physical quantities of particles, such as The position x and momentum p can be predicted from the initial conditions.
Uncertainty Principle
It is impossible to measure both position and momentum of a particle at the same time with perfect accuracy. There is always a fundamental uncertainty associated with any measurement. This uncertainty is not due to the measuring instrument. This uncertainty is completely negligible for the position and momentum measurement of macroscopic objects. But for microscopic objects, this fundamental uncertainty is the main fact.
Heisenberg uncertainty principle
In 1927 Werner Heisenberg proposed a principle based on the wave-particle duality of matter and radiation and hence known as the Heisenberg uncertainty principle.
This principle states that for particles that have both particle and wave nature, it will not be possible to accurately determine both the position and velocity at the same time.
Uncertainty Principle Example:
A stream of light photons scattering from a flying tennis ball hardly affects its path. But when a photon strikes an electron, it affects the motion of the electron. Since light has wave properties, we can determine the position of the electron only within one wavelength of the light being used. Hence for less uncertainty in position and for minimum diffraction effect, we use the light of short wavelength.
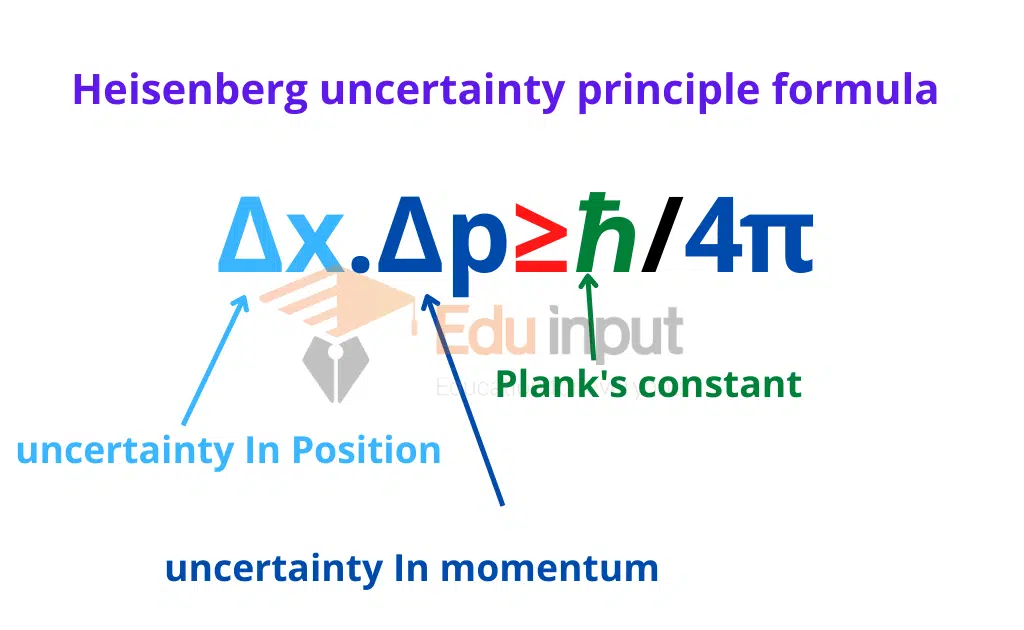
Uncertainty Principle formula
Uncertainty in position measurement of a microparticle moving along the x-axis is of the order of the wavelength λ of the light used.
Δx≈ λ
Now the exact value of change in momentum of a microparticle cannot be measured. The photon of light can transfer all its momentum h/ λ to a microparticle. Thus the change in momentum of the microparticle is of the order of h/ λ of the momentum of photons.
ΔP≈ h/ λ
It is clear if light has a shorter wavelength there is accuracy in position measurement but uncertainty in momentum. On the other hand if light has a large wavelength there is uncertainty in position but certainty in the momentum of the microparticle.
Multiplying both uncertainties
Δx .ΔP ≈ λ (h/ λ) ≈ h
This is the mathematical form of the uncertainty principle. It states that the product of the uncertainty in position Δx of a particle and uncertainty in its momentum ΔP at the same time will be approximately equal to plank’s constant (h).
Second Statement:
The product of the uncertainty in measuring the energy and the time interval during which it is measured is approximately equal to the plank’s constant (h).
ΔE = hΔf
ΔE ≈h .1/ Δt
ΔE . Δt ≈h
If ΔE is the uncertainty in the energy of a particle and the time interval during which the particle had the energy E ± ΔE/2 is t ± Δt/2
ΔE. Δt ≈h
Thus more accuracy in the energy of a particle the more uncertain the time. According to Heisenberg’s more careful calculation found that
Δx. ΔP≥ h
ΔE .Δt≥ h
h=h/2π =1.05*10-34jsce
Werner Karl Heisenberg received the noble prize for physics in 1932 for the development of quantum mechanics.





Leave a Reply