Uranium-Discovery, Properties, and Applications
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. Uranium is a naturally occurring element and is found in small amounts in rocks, soil, and water. It has several isotopes, but U-238 and U-235 are the most important for their nuclear properties.
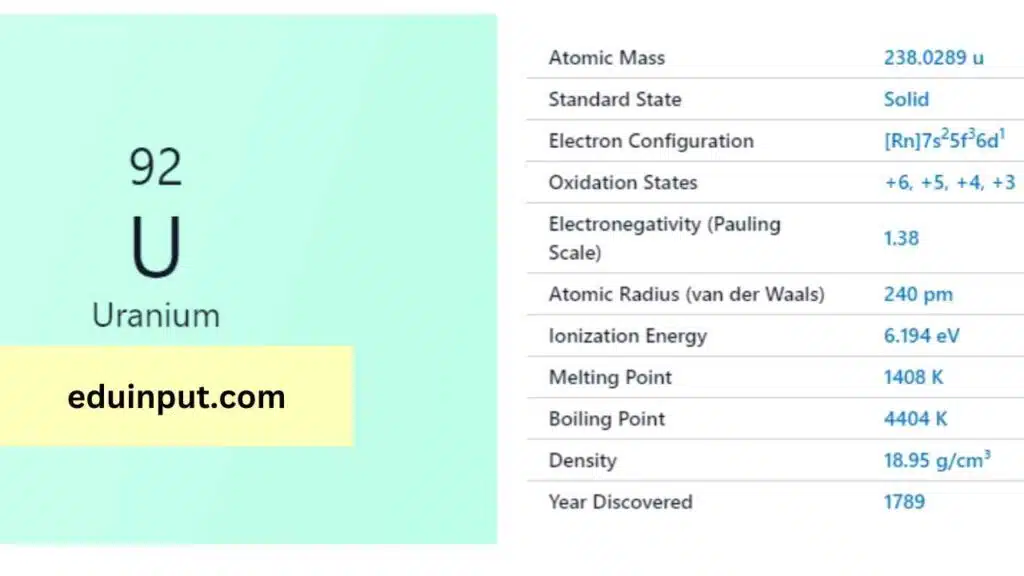
| Property | Value |
| Name | Uranium |
| Symbol | U |
| Atomic number | 92 |
| Relative atomic mass (Ar) | 238.02891 (3) [see notes g m |
| Standard state | Solid at 298 K |
| Appearance | Metallic grey |
| Classification | Metallic |
| Period in the periodic table | |
| Group name | Actinoid |
| Block in the periodic table | 7 (actinoid) |
| Block in periodic table | f |
| Shell structure | 2.8.18.32.21.9.2 |
| CAS Registry | 7440-61-1 |
Discovery
Uranium was discovered in 1789 by German chemist Martin Heinrich Klaproth. He named it after the planet Uranus, which had been discovered eight years earlier. Uranium was first isolated in 1841 by French chemist Eugène-Melchior Péligot.
Physical Properties
Uranium is a heavy, silvery-white metal that is malleable, ductile, and slightly paramagnetic. It has a melting point of 1132°C and a boiling point of 3818°C. Uranium is a dense metal and is radioactive, with a half-life of about 4.5 billion years.
Chemical Properties
Uranium is a reactive metal and reacts with most non-metal elements. It oxidizes slowly in air to form a black oxide coating. It is a relatively strong reducing agent and reacts with water to produce hydrogen gas. Uranium is used in nuclear reactors to generate energy by nuclear fission.
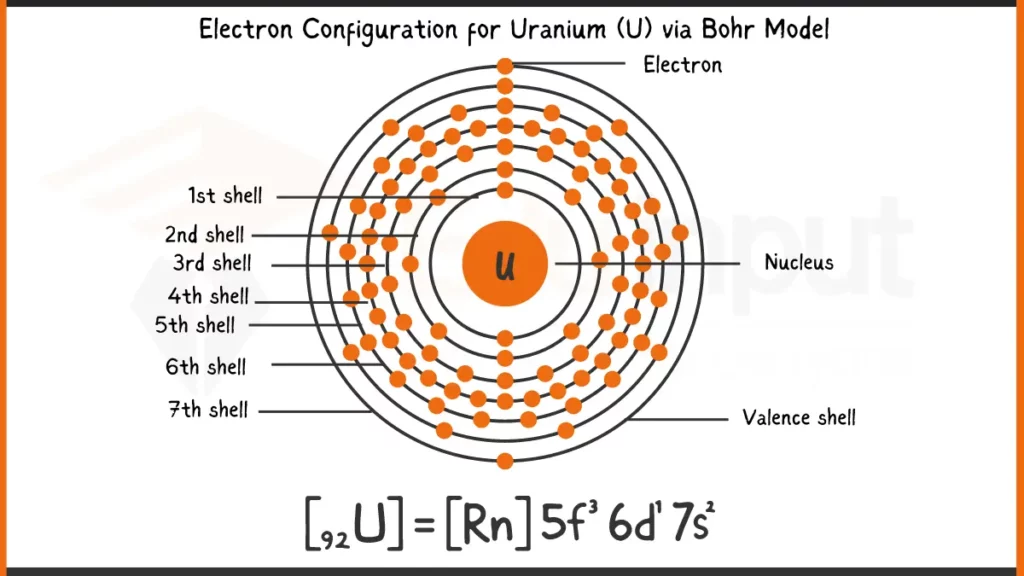
Electronic Configuration of Uranium
Uranium (U), atomic number 92, boasts 92 electrons. Its configuration is [Rn] 5f³6d¹7s², reflecting the filling of its electrons after Radon’s full shells. This shorthand indicates 3 electrons in the 5f subshell, 1 in 6d, and 2 in the outermost 7s subshell for optimal stability.
Electronic Configuration of Uranium via Bohr Model
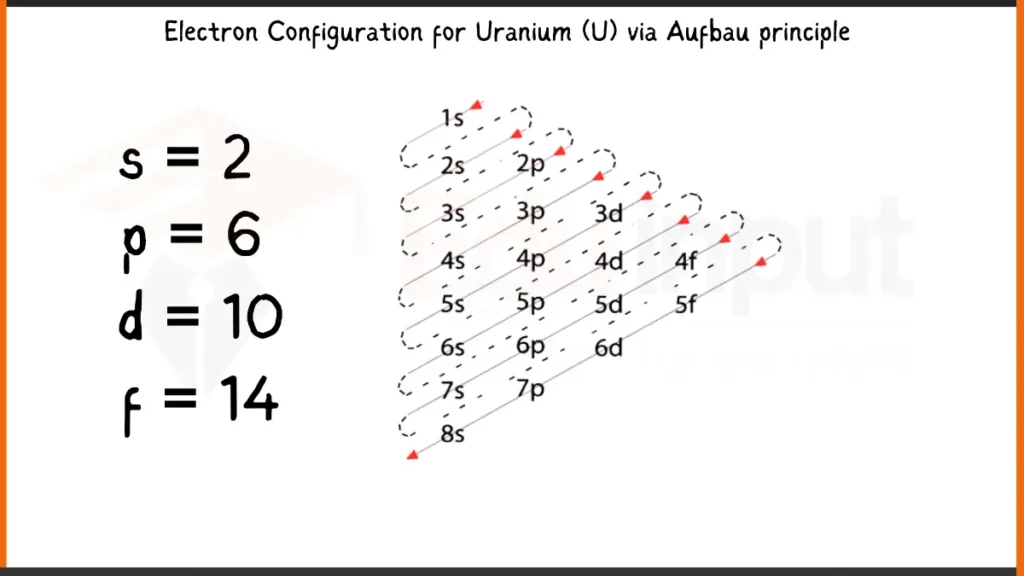
Electronic Configuration of Uranium via Aufbau Principle
Facts
- Uranium is used in nuclear weapons and nuclear power plants.
- The most common isotope of uranium, U-238, makes up more than 99% of natural uranium.
- Uranium has 26 known isotopes, with atomic masses ranging from 217 to 242.
- Uranium was once used as a yellow pigment in glass and ceramics, but this use has declined due to its radioactivity.
Applications
- Nuclear power generation
- Military applications (nuclear weapons)
- Medical applications (radiography and radiation therapy)
- Industrial uses (smoke detectors and gauges)
Uranium is a highly versatile and useful element with a range of applications in energy, medicine, industry, and military. Its radioactive properties make it both powerful and dangerous, but with proper handling and disposal, it can be used safely and effectively. As we continue to seek alternative sources of energy, the importance of uranium as a fuel source for nuclear reactors may become even more significant.





Leave a Reply