Valence Shell Electron Pair Repulsion Theory
Introduction
This theory was put forward by Sidgwick and Powell in 1940. They said that the shapes of the molecules could be interpreted in terms of electron pairs in the outer orbit of the central atom.
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used in chemistry to predict the molecular geometry of a molecule based on the idea that electron pairs, whether bonding or non-bonding, will arrange themselves around a central atom in a way that minimizes their repulsion. The theory assumes that electron pairs, whether in bonds or as lone pairs, repel each other, and the molecular geometry adjusts to achieve the maximum distance between these electron pairs. VSEPR theory is particularly useful in predicting the shapes of molecules and the angles between bonds in simple molecules.
Recently, Nylholm and Gillespie developed the VSEPR theory, which can explain the shape of molecules for non-transition elements.
Basic Assumptions of VSEPR Theory
The fundamental idea in this theory is that the valence electron pair around the central atom arranges themselves to give a geometry to the molecule. These valence electrons may be lone pairs or bond pairs. These electron pairs remain at a maximum distance apart to keep the repulsions at a minimum.
Postulates of VSEPR Theory
i) The lone pairs and the bond pairs both participate in determining the geometry of molecules.
ii) The electron pairs arrange around the central polyvalent atom and remain at a maximum distance apart to avoid repulsion.
iii) The electron pairs in the form of lone pairs occupy more space than the bond pairs.
iv) The magnitude of the repulsion between the electron pairs in a given molecule decrease in the following order.
Lone pair- lone pair > lone pair-bond pair > bond pair-bond pair.
v) These repulsions are called van der Waal’s repulsions.
vi) The two electron pairs of a double bond and three electron pairs of a triple bond contain higher electronic charge density. For this reason, they occupy more space than one electron pair of a single bond.
vii) While determining the geometry of the molecule, the two electron pairs of a double bond and three electron pairs of a triple bond, behave like a single bond. The reason is that they tend to occupy the same region between the two nuclei like a single bond.
Reason for More Repulsion of Lone Pair than Bond Pair
A bonding electron pair is attracted by both nuclei of the atoms. A non-bonding electron pair is attracted by only one nucleus. Lone pair experiences less nuclear attraction, so its electronic charge is spread out more in space than that of a bonding pair So the non-bonding electron pair exerts greater repulsive forces on bonding electron pairs. In this way, they tend to compress the bond pair.
Types of Molecules in VSERP Theory
In order to illustrate the VSEPR theory, let us consider that the central atom is ‘A’ and this atom is polyvalent. More than one ‘B’ type of atom is linked with ‘A’ to give AB2, AB3, AB4, AB5, AB6, and AB7 type molecules. It depends upon the valency of the atom ‘A’ and how many ‘B’ types of atoms are attached to that. The following table gives the shapes of different types of molecules.
Geometries of molecules
1. Molecules containing two electron pairs; (AB2) type
In these molecules, a central atom ‘A’ can manage two unpaired electrons in its outermost shell. The ‘B’ type atoms can share their electrons to make two covalent bonds. In this way, two bonding electron pairs are developed and they arrange at 180° to each other. In this way, they can minimize the repulsion between them. They are said to have linear geometry.
Examples:
The elements of group II-A have two electrons in the outermost orbitals. When they combine with monovalent atoms like F, Cl, Br, I, etc. then they give linear structures. These molecules are BeCl2, MgCl2, CaCl2, SrCl2, BaCl2 etc. Similarly, their bromide and behave accordingly.
- Cl-Be-Cl
- Cl-Mg-Cl
- Cl-Sr-Cl
Similarly, the elements of group II-B i.e. Zn, Cd, and Hg also have a structure with halogens. These compounds are ZnCl2, CdCl2, and HgCl2.
- Cl-Zn-Cl
- Cl-Cd-Cl
- Cl-Hg-Cl
2. Molecules containing three electron pairs; (AB3) type
In these molecules, the central atom ‘A’ can have three unpaired electrons. In these free monovalent atoms ‘B’ join with ‘A’ to give three bonding electron pairs. If the atom ‘A’ has four electrons in the outermost orbital, then three electron pairs can be with one lone pair and two bond pairs.
(a) AB3, Type with No Lone Pair
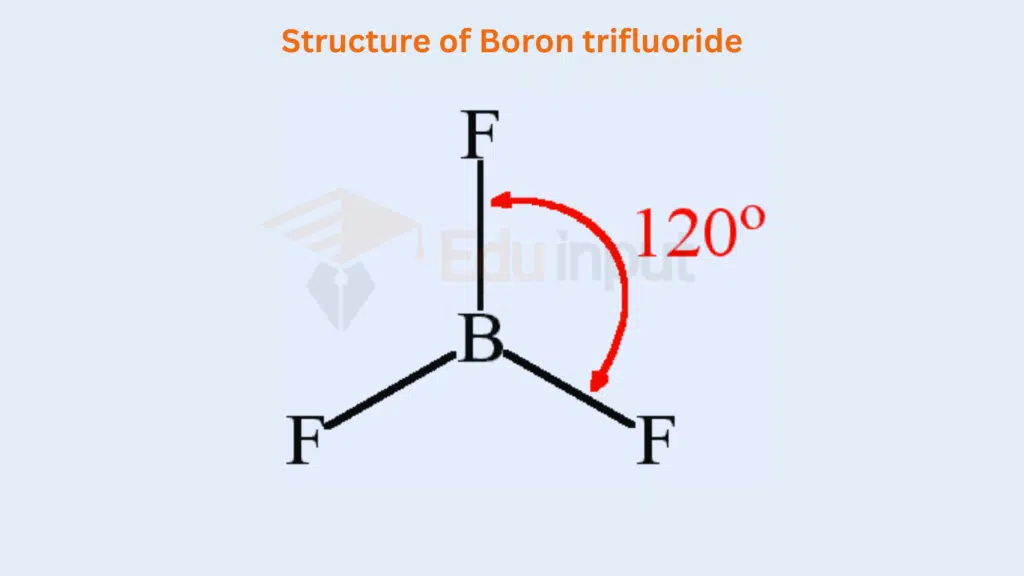
The central atom ‘A’ contains three bonding electron pairs in these molecules. They arrange at a maximum distance apart and create an angle of 120°. In this way, a triangular planar structure is developed. The elements of group III-A i.e. B, Al, Ga, In, and Tl, when combined with monovalent atoms like H, F, CI, Br and give such compounds. Various compounds are as follows, BH3, BF3, AICI3, etc.
(b) AB3, type with one lone pair and two bond pairs
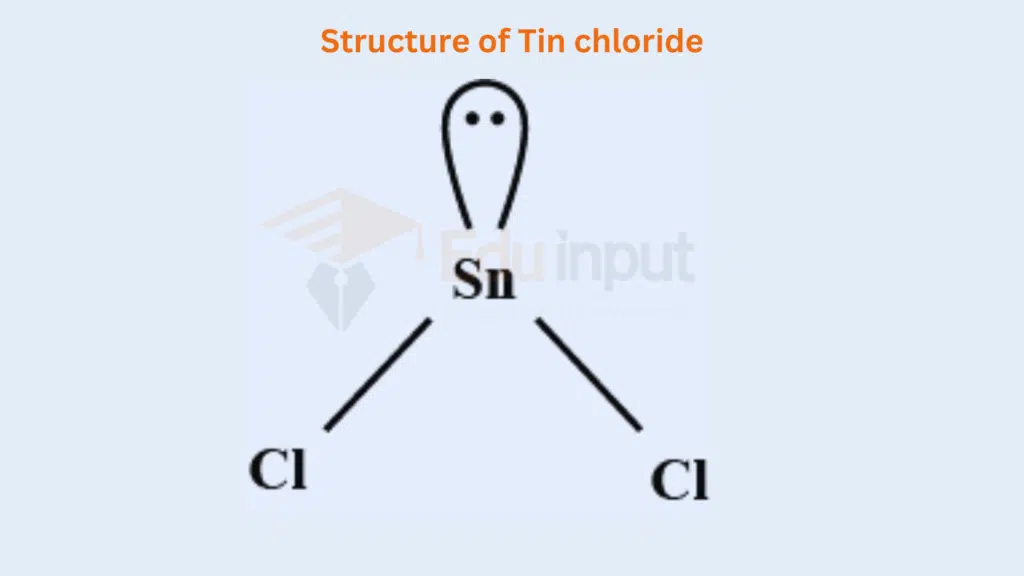
The elements Sn and Pb are present in the IV-A group of the Periodic Table. They are four electrons in the outermost orbital. Due to the inert pair effect, they do not promote the electron from the s orbitals to the p orbital. So, they show the valency of two, in making SnCl2 and PbCl2. They manage one lone pair in the outermost shell and have the following geometries.
The bond angle becomes less than 120°, due to the greater repulsion of a lone pair on the bond pairs.
(c) AB3, type with multiple bonds
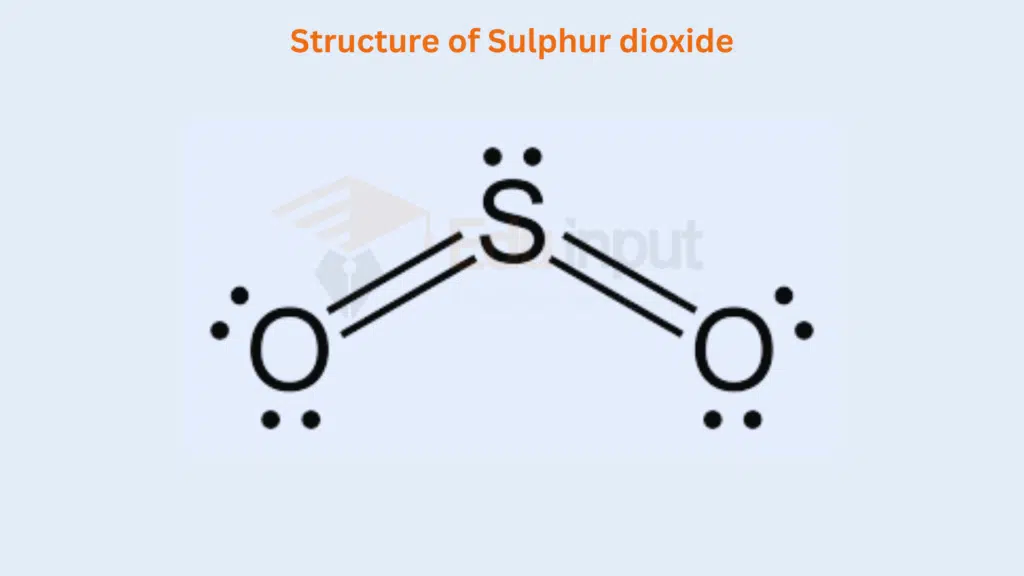
In the molecules of SO2, one comer of the triangular is occupied by lone pair and two comers each by the S=O group.
In SO3 molecule all three regions are occupied by S=O bonds. There is no lone pair around the Sulphur atom. So, the structure of SO3 is perfectly triangular.
3. Molecules containing four electron pairs, (AB4) type
Depending upon the number of lone pairs and the bond pairs around the central atom ‘A’ there are three situations. Let us discuss them one by one.
(a) AB4, type with no lone pair
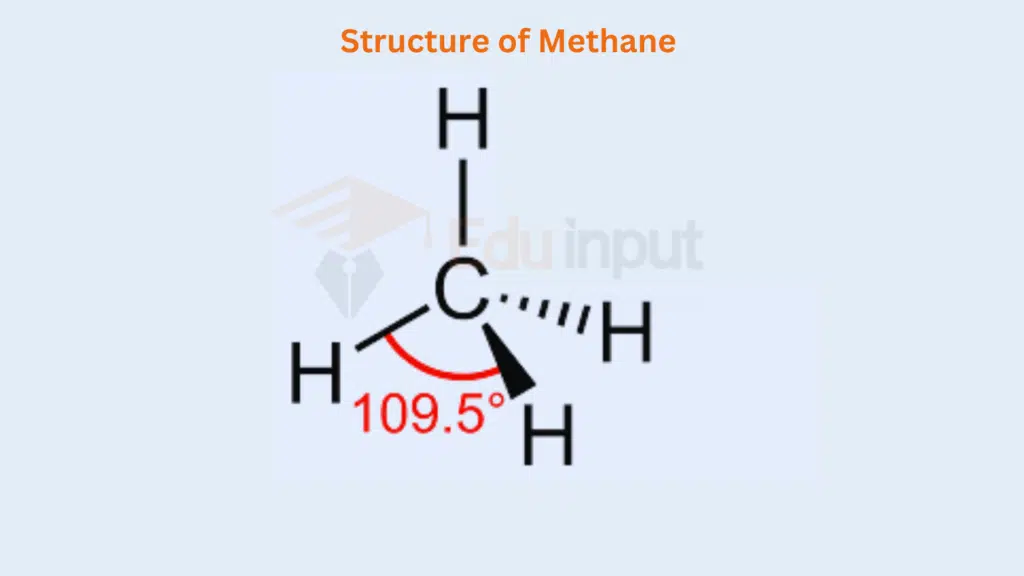
In such compounds, all the electron pairs. They repel each other in a symmetrical manner and maintain an angle of 109.5° and a regular tetrahedral geometry is developed. A tetrahedron has four corners, six edges, and six bond angles. The structure is as follows:
Examples:
The elements in group IV-A have four electrons in the outermost shells. They share these four electrons with four monovalent atoms as H, F, Cl, Br, and I, and give the compounds like CH4, SiH4, CCl4, SiCl4, GeCl4, etc.
(b) AB4, type with one lone pair and three bond pairs
If one of the lone pairs is present around the central atom in AB4-type compounds then the bond angle decreases due to the greater repulsion of the lone pair on the bond pair. The following type of geometry is developed which is a little distorted tetrahedral.
Examples:
The elements of groups V-A are N, P, As, Sb, and Bi. When they react monovalent atoms like H, F, Br, Cl, and I, etc., give such types of compounds. In the structure of NH3, the non-bonding electron in the 2s orbital takes up more space and exerts a strong repulsive force on the bonding electron pairs. The structure of NH3 is as follows. The lone pair decreases the angle from 109.5° to 107.5°.
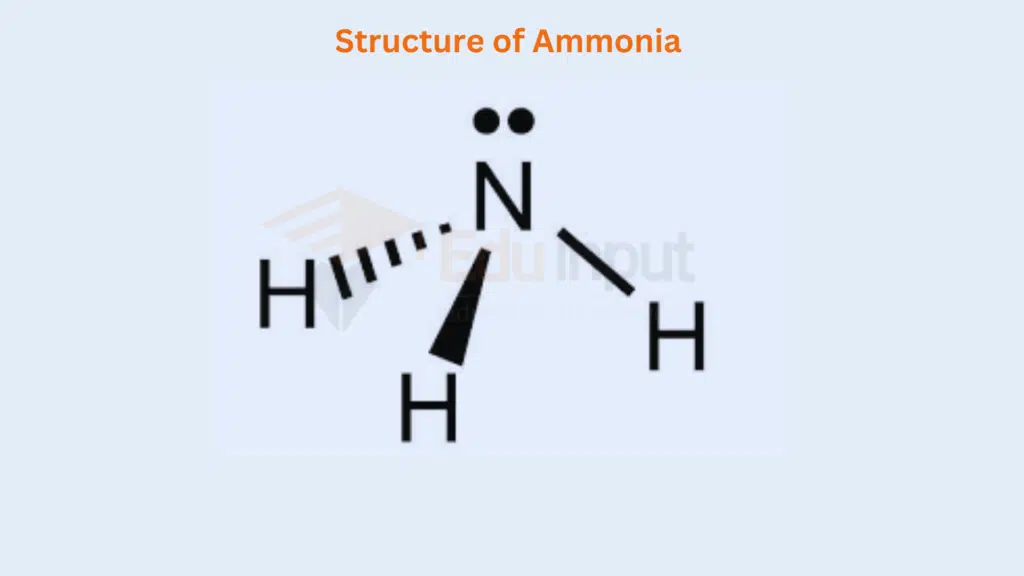
On similar grounds, the structures of PH3, AsH3, SbH3, and BiH3 can be justified. But, keep in mind that the bond angles of these molecules are less than that of NH3. This is due to greater sizes of the central atoms and greater bond lengths.
Structure of NF3:
This is an AB4-type compound just like NF3 having a lone pair on the nitrogen atom. The lone pair of nitrogen is pulled closer to its nucleus. In this way, it exerts a stronger repulsion over the bonding electrons. The bond angle further decreases and shrinks up to 102°. Moreover, the bond pairs of Nf bonds are closer to the F atoms than nitrogen atoms. In this way, the distances between the bond pairs increase, and their mutual repulsion decrease. For this reason, the bond angles decrease.
(c) AB4 type with two lone pairs and two bond pairs
If the central atom has two lone pairs and two bond pairs, then the geometry of the molecule is bent and only one bond angle is there. The four electron pairs are arranged in a tetrahedral manner, but the molecule seems to be AB2 type.
Examples:
The elements of group VI-A as O, S, Se, Te, and Po can do this job. When combine with monovalent atoms like H and halogens, then they show bent structures.
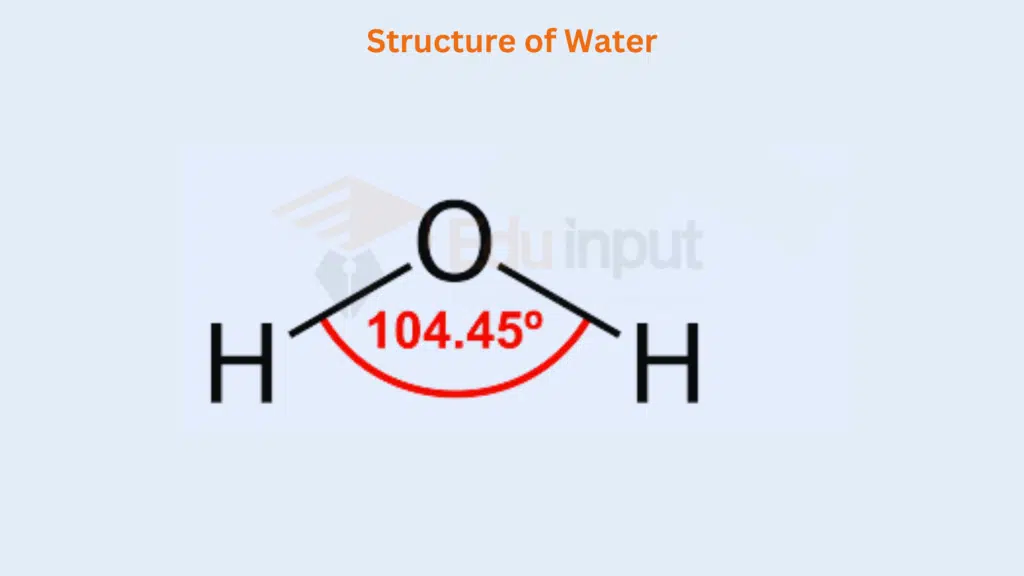
Two of the comers of the tetrahedron are occupied by each of the two lone pairs, and the remaining by the bond pairs. The bond angle in the H2O molecule decreases from 109.5° to 104.5″. Similarly, the bond angles in H2S, H₂Se, and H₂Te are even less than this.
4. Molecules containing five electron pairs, (AB5) type
When the central atom has five unpaired electron, then they arrange in a triangular pyramid geometry. Three electrons pairs are in one plane while other two are perpendicular to the plane. The best example in this reference is PCl5.
5. Molecules Containing Six Electrons Pairs (AB6, type)
When the central atom has six electron pairs then they arrange in a square bipyramid geometry. Four electron pairs are in one plane while two are perpendicular to that. The best example in this reference is SF6.
Limitation of VSEPR Theory
- It fails to explain how atoms share electron in their valence shell.
- It does not explain bond energies and bond length.
- It does not give reasons for the formation of bonds.






Leave a Reply