What are Organic Molecules? | Properties of Organic Molecules
Have you ever wondered what an organic molecule is? Do you know what it looks like or what functions it has? Organic molecules are examples of chemical compounds that contain carbon, hydrogen, and other elements in the organic group. Although they may seem complex at first glance, these molecules are often simpler than they appear.
Organic compounds are ubiquitous and play a wide role in the life of living organisms. They are essential for all existing forms of life.
These compounds can also be found in pharmaceuticals, fragrances, cosmetics, industrial chemicals, food additives, essential oils, pesticides, agricultural products, petroleum fuels, and explosives. They’re so common because natural organic compounds are easy to manufacture from simple substances found in the environment.
Basic Characteristics of Organic Molecules
- Organic molecules are carbon-based, large, and complex.
- They have a distinctive odor and can be classified by their functional groups.
- They have a low melting point.
- They have a low boiling point.
- They show low solubility in water but high solubility in nonpolar solvents.
- They can be flammable or nonflammable.
- They do not conduct electricity in aqueous solutions.
- They exhibit covalent bonding.
Examples of Organic Molecules
- Methane (CH4)
- Lipids (Triglycerides)
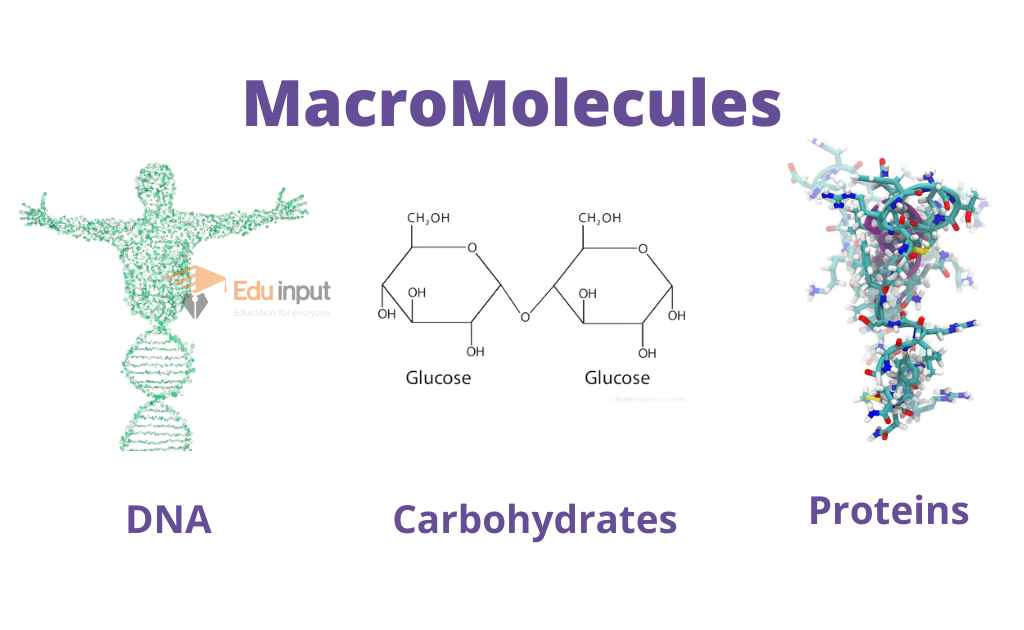
- Nucleic Acids (DNA)
- Proteins
Covalent Bond Formation
Carbon can also form covalent bonds with other carbon atoms, as well as with atoms of other elements including hydrogen, oxygen, and nitrogen. This is called heterocyclic chemistry because it involves the formation of molecules or polycyclic compounds.
The complexity of organic molecules
- Most organic compounds are large and complex molecules containing dozens or hundreds of atoms.
- Molecules are made up of atoms, which can be either positively charged (cat-ions) or negatively charged (an-ions).
Functional Group
The functional group is an atom or group of atoms that give specific properties to the molecule. Functional groups are important in determining the reactivity of a molecule, as well as its biological and physical properties. These include:
- Hydrogenation: This reaction occurs when one functional group reacts with another to form a new substance
- Nucleophilic substitution: When a nucleophile attacks an electrophile (a species that can donate electrons), it forms new bonds between them and rearranges their structure
Functional Groups of Organic Molecules
| Functional group | Properties |
| Hydroxyl | Polar |
| Methyl | Non polar |
| Carbonyl | Polar |
| Carboxyl | They are considered acidic,as it , ionizes to release H+. |
| Amino | Accepts H+ |
| Phosphate | They are considered acidic,as it , ionizes to release H+. |
| Sulfhydryl | Polar |
Bases Of Classification Of Organic Molecules
Most organic compounds can be classified by their functional groups, which are responsible for different chemical reactions. Functional groups are small molecules that serve as the dots in a molecule’s structure.
For example, the alkene group is responsible for the unique properties of an alkyl radical and so acts as a kind of gateway to other functional groups.

 written by
written by 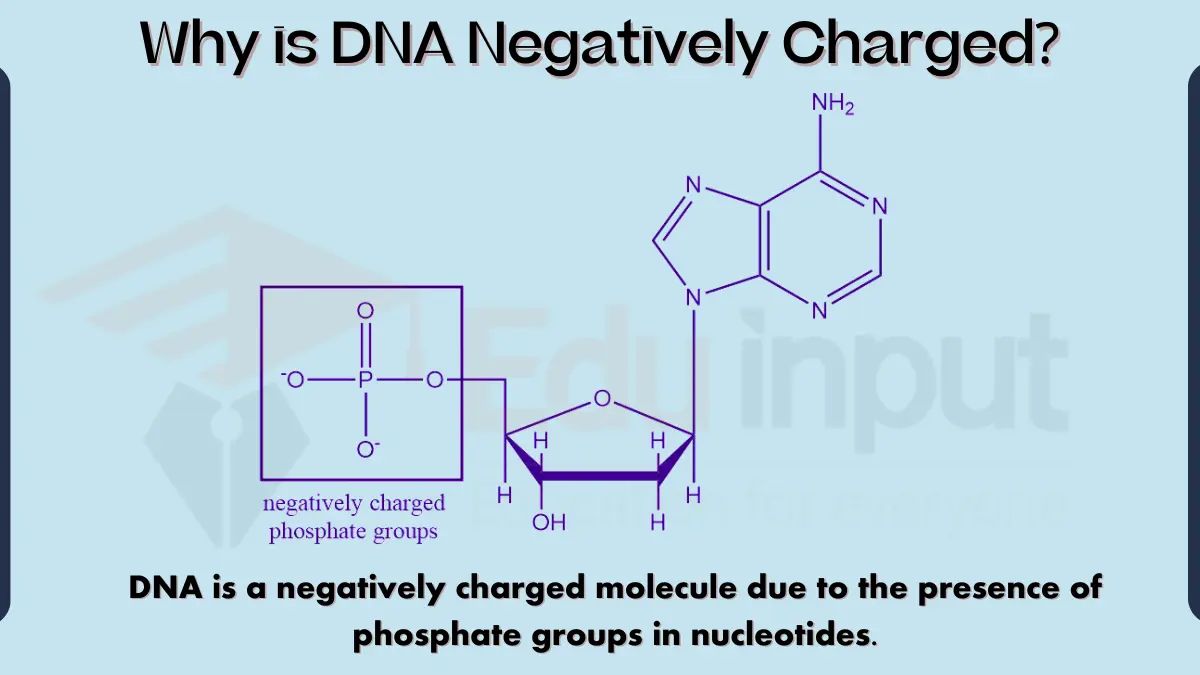


Leave a Reply