What is Internal Energy-Definition And Example
The sum of all forms of molecular energies (kinetic energy and potential energy) of a substance is called Internal Energy.
Internal energy
In thermodynamics, we usually take the ideal gas as a working substance. As the molecules of an ideal gas are point masses, so they exert no force on one another. Thus the internal energy of an ideal gas system is generally the translational K.E of its molecules.
Since the temperature of a system is the average K.E of its molecules. Therefore, for an ideal gas system, the internal energy is directly proportional to its temperature.
Internal energy is a broad property. A thermodynamic process that defines internal energy is the transfer of matter or energy as heat or thermodynamic work. These processes are measured by changes in a wide range of system variables, such as entropy, volume, and molar composition. In many cases, it is not necessary to consider all the energies of the system itself, such as the static rest mass energies of its constituent materials.
A system is said to be closed if the movement of matter is impeded by an impermeable enclosing wall, and the first law of thermodynamics dictates that the change in internal energy is the energy input into the system as heat, and the system is its local area.
A system is said to be isolated if the surrounding walls do not allow matter or energy to pass through it, and its internal energy cannot change.
How to change internal energy
When a substance is heated, the energy of its atoms increases. It means that the heat is converted into internal energy. But internal energy can also be increased without giving heat to the system, such as when two bodies are rubbed, their internal energy is increased due to the work done. When an object slides over any surface and comes to rest due to frictional forces, the mechanical work done on the system or by the system is partially converted into internal energy.
Independent of path:
Internal energy does not depend on the path but it depends on the initial and final states of the system.
Internal energy Example
Consider a system that changes from Pa, and Va, to Pb, and Vb, such that.
Pa =Initial Pressure
Pb=Final Pressure
Va=initial volume
Vb=final volume
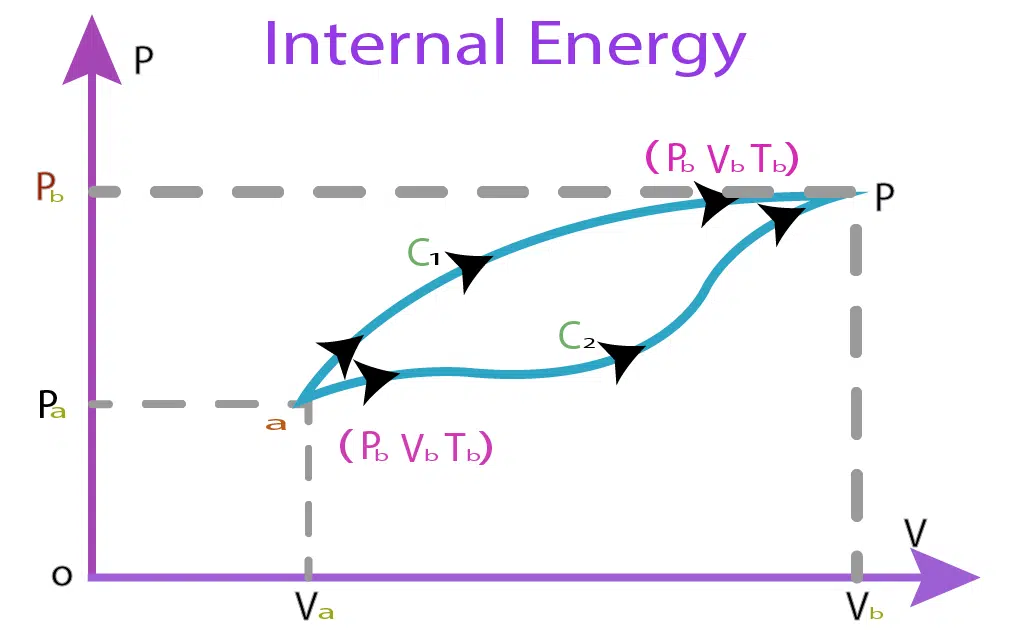
Experimentally it has been proved that the change in internal energy remains the same and does not depend on paths C1 and C2. The internal energy of a system is denoted by ‘U.’ Like gravitational P.E., we always take the change in internal energy and not its absolute value.
ΔU=Ub – Ua
Ua=initial internal energy
Ub=final internal energy.





Leave a Reply