What is pH?-Definition, Calculation, Applications, And Limitations
Definition of pH
pH is a measure of the acidity or alkalinity of a solution. It indicates how many hydrogen ions (H+) are present in the solution. The pH of a solution depends on the relative amounts of acids and bases it contains.
Acids increase the concentration of hydrogen ions, while bases reduce hydrogen ion concentration by accepting protons. Strong acids, like hydrochloric acid, tend to have lower pH values around 0-3. Strong bases, like sodium hydroxide, usually have higher pH values around 13-14. Pure water has a neutral pH of 7.
How to calculate pH?
The pH of a solution can be calculated using the formula:
pH = -log[H+]
Where [H+] is the concentration of hydrogen ions in moles per liter.
To find [H+], you can use the dissociation of water equation:
H2O ⇌ H+ + OH–
In pure water at 25°C, the concentrations of H+ and OH- are equal at 1 x 10-7 M.
So for pure water: pH = -log(1 x 10-7) = 7
For an acidic solution with a hydrogen ion concentration of 1 x 10-4 M:
[H+] = 1 x 10-4 M pH = -log(1 x 10-4) = 4
So this acidic solution would have a pH of 4.
The lower the pH, the higher the hydrogen ion concentration and acidity. Higher pH solutions have less hydrogen ions and are more alkaline. By calculating pH, chemists can characterize the properties of aqueous solutions.
What is the pH Scale?
The pH scale is a measurement scale used to express the acidity or alkalinity (basicity) of a solution. It quantifies the concentration of hydrogen ions (H⁺) in a solution. The pH scale typically ranges from 0 to 14, with 7 as the midpoint or neutral point.
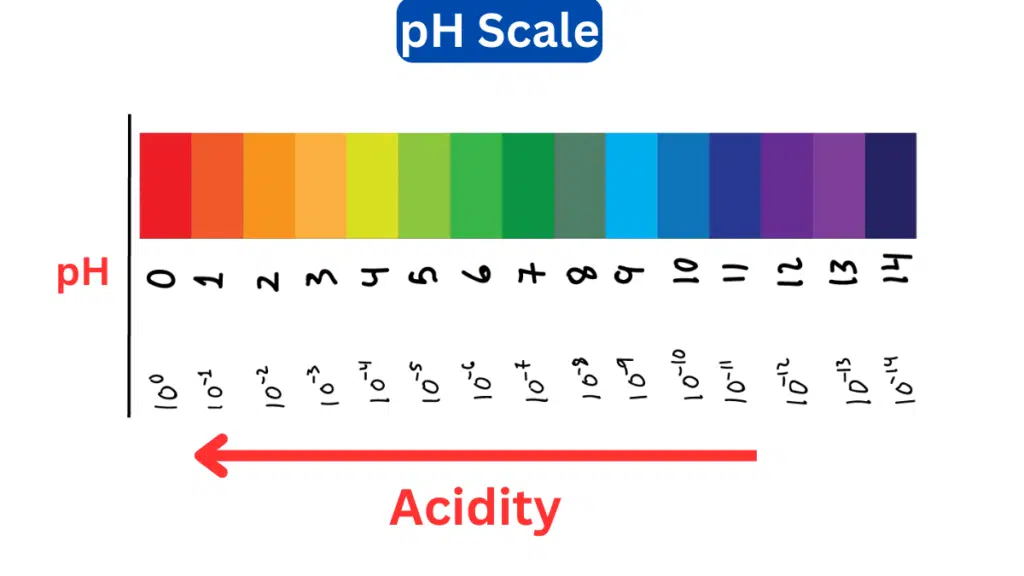
Here’s a breakdown of how the pH scale works:
- Acidic Solutions (pH < 7): Solutions with a pH lower than 7 are considered acidic. The lower the pH value, the more acidic the solution. For example, substances with a pH of 1 or 2 are very acidic, like stomach acid or lemon juice.
- Neutral Solution (pH = 7): A pH of 7 represents neutrality. Pure water at room temperature is typically considered neutral because it has an equal concentration of hydrogen ions (H⁺) and hydroxide ions (OH⁻).
- Basic or Alkaline Solutions (pH > 7): Solutions with a pH greater than 7 are considered basic or alkaline. The higher the pH value, the more basic the solution. For instance, substances with a pH of 12 or 13 are highly alkaline, such as bleach or sodium hydroxide.
pH of Different Substance
Here is a table showing the pH values of 20 different substances:
| Substance | pH Range | Description |
| Stomach acid | 1-3 | Highly acidic, aids in digestion. |
| Lemon juice | 2-3 | Sour and acidic, commonly used in cooking. |
| Vinegar | 2-4 | Acidic, used in cooking and cleaning. |
| Soda | 2-4 | Carbonated soft drink, mildly acidic. |
| Beer | 4-5 | Alcoholic beverage, slightly acidic. |
| Rainwater | 5-6 | Slightly acidic due to dissolved gases. |
| Milk | 6-7 | Slightly acidic to neutral, a dairy product. |
| Pure water | 7 | Neutral, neither acidic nor basic. |
| Blood | 7.35-7.45 | Slightly basic, vital for bodily functions. |
| Seawater | 8 | Moderately basic, found in oceans. |
| Baking soda | 8-9 | Basic, used in baking and as an antacid. |
| Egg whites | 8 | Slightly basic, used in cooking and baking. |
| Milk of magnesia | 10-11 | Strongly basic, used as an antacid and laxative. |
| Ammonia | 11-12 | Strongly basic, used in cleaning and industry. |
| Bleach | 12-13 | Highly basic, used as a disinfectant and cleaner. |
| Soapy water | 12-13 | Highly basic, soaps are alkaline in nature. |
| Oven cleaner | 13-14 | Extremely basic, used to remove oven residue. |
| Drain cleaner | 13-14 | Extremely basic, used to unclog drains. |
| Sodium hydroxide | 14 | Highly basic, a strong alkaline compound. |
| Limewater | 12-13 | Slightly basic, used in laboratories and industry. |
| Coffee | 5-6 | Slightly acidic to neutral when brewed. |
Importance of pH scale
Here are some of the key reasons why pH is important:
- Biochemistry – Enzyme function and protein structure are highly dependent on pH. Cells and bodily fluids must maintain a pH within a narrow range to support life.
- Medicinal applications – The pH of solutions affects the absorption of drugs and delivery of compounds to targets in the body. Controlling pH allows the optimization of medications.
- Food preservation – The acidity of foods helps prevent microbial growth and spoilage. Adjusting pH through pickling, fermentation, or acidulants extends shelf life.
- Cleaning and disinfection – Many cleaning agents and disinfectants work by altering pH. Their germicidal efficacy depends on pH levels.
- Water chemistry – The pH of natural waters influences the solubility and availability of nutrients. Aquatic organisms are adapted to specific pH ranges.
- Corrosion control – Changes in pH cause metals to corrode. Monitoring pH allows prevention of costly damage to pipes, reactors, and industrial equipment.
- Acid-base chemistry – The pH scale is used universally to characterize acid and base solutions. It enables comparison of the relative acidities of compounds.
- Titrations – pH indicators allow chemists to accurately monitor pH changes during acid-base titrations to determine unknown concentrations.
- Environmental impact – Shifting pH due to industrial pollution or acid rain disrupts ecosystems. Measuring pH monitors and mitigates human environmental influence.
- Solubility – The solubility of many inorganic and organic compounds depends significantly on the pH of the solution.
Precise control and measurement of pH is critically important across scientific disciplines including medicine, biology, environmental, food, and chemical sciences.
Applications of pH scale
Here are some important applications of the pH scale:
- Medicine – The human body requires a tightly controlled pH for proper enzyme function and overall health. Doctors and scientists use pH measurements to monitor blood, urine, saliva, and other bodily fluids. Abnormal pH levels can indicate disease or improper function.
- Food Science – The pH of foods affects safety, taste, and texture. Food scientists measure pH during processing, storage, and preparation to ensure quality. Acidity prevents bacterial growth in foods like pickles and fruit juice.
- Water Quality – Natural waters have a pH range necessary for animal and plant survival. Pollutants from acidic rain or alkaline wastes can harm ecosystems if the pH shifts too far. Monitoring pH allows the protection of water resources.
- Aquariums – Maintaining proper pH is crucial for healthy aquatic life in aquariums and fish tanks. pH affects the toxicity of ammonia from waste products. Most aquarium species require a specific pH range.
- Agriculture – Soil pH indicates nutrient availability for crops. Farmers may adjust soil acidity with lime or sulfur to optimize conditions for plant growth. pH also affects the action of fertilizers and pesticides on crops.
- Swimming Pools – Pool water must be kept within an ideal pH range to balance disinfectant effectiveness and prevent harm to swimmers. Pool pH is carefully monitored and adjusted.
- Chemical Engineering – The pH of raw materials, reactants, and finished products is monitored to ensure quality. The pH scale allows engineers to characterize acidity and alkalinity.
- Environmental Science – pH is an important indicator of pollution in ecosystems like lakes and forests. Acid rain causes environmental damage by lowering natural water and soil pH.
- Chemistry – Chemists use pH measurements in almost every aqueous reaction to understand conditions. The pH scale provides a universal way to characterize acidity and basicity.
Limitations of pH Scale
Here are some key limitations of the pH scale:
- Narrow range – The pH scale only ranges from 0 to 14, which limits its usefulness for very concentrated acid and base solutions. Extreme pH values beyond this range cannot be measured.
- Temperature dependence – The pH of a solution changes with temperature, as the water dissociation equilibrium is temperature-dependent. Values are only standard at 25°C.
- Does not account for acid or base strength – pH only measures hydrogen ion concentration, not the relative strengths of acids or bases. Strong and weak acids at the same pH can have very different properties.
- Measurement challenges – Precise pH measurement can be complicated by electrode junction potentials, calibration errors, and interference from other ions. Results may not always be completely accurate.
- Non-aqueous solutions – The pH concept only applies well to aqueous solutions. Different approaches are needed for non-aqueous solvents or molten salts.
- Kinetic limitations – The pH represents an equilibrium state, but reactions may be kinetically slow to reach equilibrium. Measured pH may not reflect equilibrium pH.
- Buffering effects – pH changes very slowly for well-buffered solutions. pH measurements may not reveal when acids/bases are added to buffered systems.
- Complex chemical effects – Some compounds hydrolyze or exhibit other equilibria that make pH hard to interpret. The pH may not reflect all chemical phenomena.
- Biological variation – The pH of biological systems like blood or soil is not uniform. A single pH value may not adequately characterize these complex matrices.
- Subjective color indicators – Color-based pH indicators provide only approximations and are subject to human interpretation. Values depend on lighting and user proficiency.
While very useful, the pH concept has limitations that must be understood when applying it analytically or in chemical reactions and processes. Multiple methods may be needed to fully understand acid-base behavior.



Leave a Reply