What is the Difference Between Bioburden and Microbial Limit Test?
Key Difference
The key difference between bioburden and microbial limit testing lies in their purpose and scope. Bioburden testing measures the total number of microorganisms present on a product before sterilization, helping to assess the effectiveness of the sterilization process.
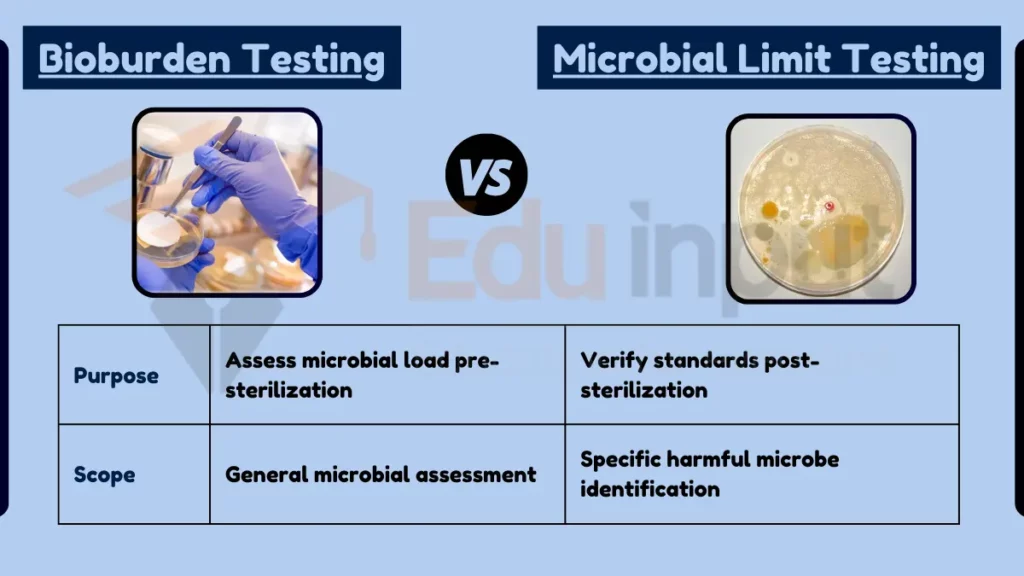
Microbial limit testing, on the other hand, is conducted post-sterilization to ensure that the product meets specific standards for microbial contamination, focusing on identifying and quantifying particular types of harmful microorganisms.
Comparative Analysis
- Purpose:
- Bioburden Testing: Determines total microbial load before sterilization.
- Microbial Limit Testing: Ensures product meets contamination standards post-sterilization.
- Scope:
- Bioburden Testing: General assessment of microbial presence.
- Microbial Limit Testing: Specific identification and quantification of harmful microbes.
Table Summary of Bioburden vs Microbial Limit Test
| Feature | Bioburden Testing | Microbial Limit Testing |
|---|---|---|
| Purpose | Assess microbial load pre-sterilization | Verify standards post-sterilization |
| Scope | General microbial assessment | Specific harmful microbe identification |
Both bioburden and microbial limit tests are crucial in ensuring the safety and effectiveness of sterilization processes, each serving a distinct role in quality control.



Leave a Reply