Calorimeter-Definition, History, Construction, Types, And Uses
A calorimeter is a device that is used to measure the heat capacity of the material and the amount of heat that produces during different electrical, physical, and chemical reactions.
Calorimeter
Calorimetry is the process of measuring the heat of chemical reactions or physical changes as well as heat capacity and is done with a calorimeter.
A simple calorimeter consists of a metal container full of water suspended above a combustion chamber and a thermometer attached to it. It is the measurement device used in the study of chemistry and thermodynamics.
History of Calorimeter
The first ice calorimeters were created by Joseph Black in 1761, and in 1780, Antoine Lavoisier used the heat from the guinea pig’s respiration to melt snow.
One of the first ice calorimeters was used in the winter of 1782 by Lavoisier and Pierre-Simon Laplace, which relied on the heat needed to melt ice to water to measure the heat released from chemical reactions.
Construction of Calorimeter
Its main part is a metallic vessel made of materials that are good conductors of electricity. A facility is available for stirring the contents of the vessel.
In order to prevent heat loss to the environment, this metallic vessel with a stirrer is kept in an insulation jacket. There is only one opening through which a thermometer can be inserted to measure the change in thermal properties.
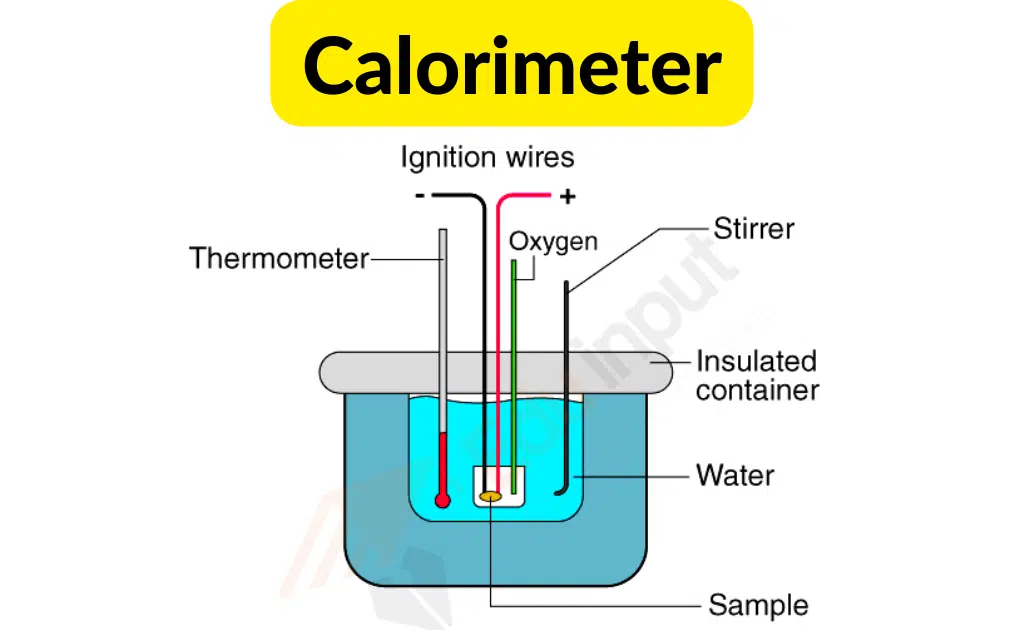
It is possible to make such measurements easily with this. In a calorimeter, there is a fixed amount of fuel burned. The vessel is filled with water, and the fuel is burned, leading to the heating of the water.
The heat loss by the fuel and the heat gained by the water is the same. In order to improve the accuracy of the experiment, it is important that the calorimeter is not exposed to the environment.
It is possible to measure the change in heat through the thermometer. It is possible to find out the heat capacity of water and the energy stored inside a fuel through this measurement.
Types of Calorimeter
The most important types of calorimeters are:
- Constant Pressure Calorimeters
- Differential Scanning Calorimeter
- Adiabatic Calorimeters
- Reaction Calorimeters
- Bomb Calorimeters
Uses of Calorimeter
Calorimetry measures the transfer and exchange of heat. It is a technique that can be used in many sectors. Physical changes of first- and second-order transitions such as glass transition, melting, and crystallization can be measured. It can be used to evaluate the parameters of thermodynamics.
Related FAQs
What is a calorimeter?
It is a device that is used to measure the heat capacity of the material and the amount of heat that produces during different electrical, physical, and chemical reactions.
What is the principle of the calorimeter?
The body at a higher temperature releases heat while the body at a lower temperature absorbs it. The principle of calorimetry is an indicator of the law of conserving energy. The total heat lost by the hot body is the same as the total heat gained by the cold body.
How calorimeter is used in real life?
Calorimetry plays a large part in everyday life, controlling the rates of metabolism in humans and maintaining temperature in the body. Calorimetry is used to measure the heat of a reaction, so it is an important part of thermodynamics.
What is the heat capacity of the calorimeter?
The heat capacity of the calorimeter is the quantity of heat absorbed by the calorimeter for each 1°C rise in temperature. The heat capacity of the calorimeter must be determined experimentally. The easiest process is to study the mixing of warm and cold water.
What is the advantage of a colorimeter?
Compared with other methods, the colorimetric method has some advantages, such as low cost, simple instruments, and can be qualitatively or semi-qualitatively identified by the naked eye. The colorimetry is less sensitive.






Leave a Reply