Classification of Solids- Crystalline Solids, Amorphous Solids, and Polymeric Solids
Classification of solids is based on the bonds that hold the atoms or molecules together. Solids are classified into the following categories
The mechanical properties of solids describe their characteristics such as their resistance to deformation and their strength.
Download the pdf notes of classification of solids class 12 physics
Crystalline solids
Molecules are regularly arranged in crystalline solids. Each molecule is attached to the other neighbor molecule exactly in the same way throughout the crystal. There is an ordered structure in crystalline solids.
Examples:
Metals such as zinc, copper, and Iron, ionic compounds like sodium Chloride, and ceramics such as zirconia are crystalline.
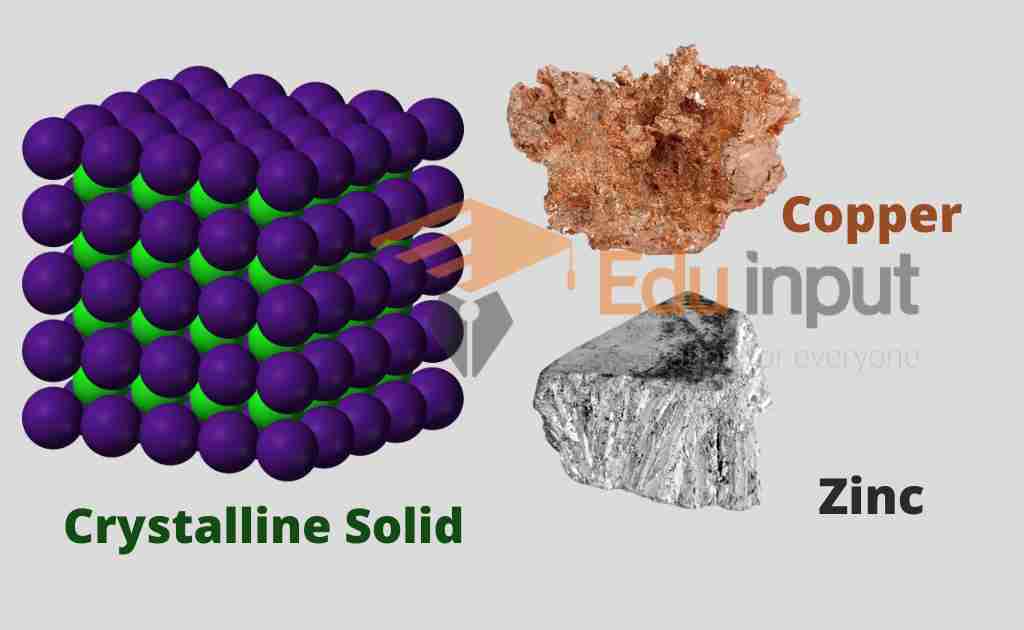
The molecular arrangement in the crystalline materials is studied with the help of X-ray technologies. The atoms, molecules, or ions are not stationary inside the crystalline solids but they do vibrate about their mean positions. When the temperature of the substance is increased, the amplitude of vibration also increases.
It is the average atomic position that is perfectly ordered over large distances.
The cohesive forces between the atoms, molecules, or ions keep the atoms in the right position despite their vibratory motion, and due to this, the crystalline solids have a regular shape.
There is an upper limit to these forces and when due to external effects, the vibrations become so large that these forces do not hold the substance together, the substance melts.
So there is a certain temperature for every solid at which it melts which is called the melting point. This new shape is rapid and irregular.
Amorphous or glassy solids
These solids are without any specific form or structure. The word Amorphous means without form or structure. It means that in amorphous solids there is no regular arrangement of the molecules. It means that amorphous solids are more like liquids with a disordered structure frozen in
Example:
Ordinary glass, which is solid at ordinary temperature, has no regular arrangement of molecules. On heating, it is melted into a paste-like state which is much more viscous at almost 800°C.
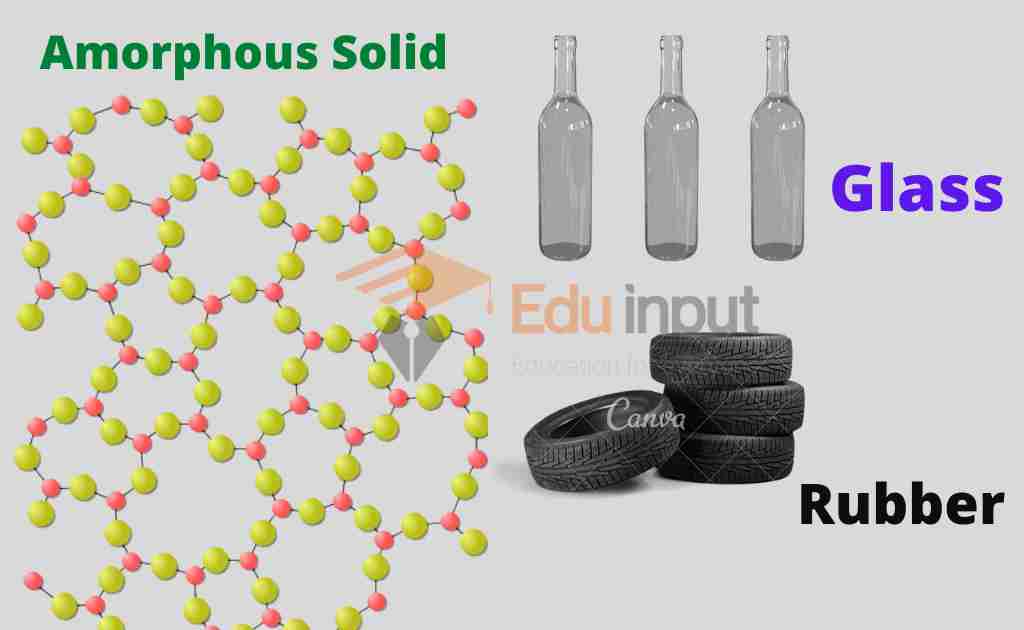
These solids are also called glassy solids. They do not have an exact melting point.
Polymeric solids
They are said to be more or less solid materials because their structure is in between an ordered (crystalline) and disordered (amorphous) structure. They can be said to be partially or poorly crystalline solids.
There are two types of polymer
- Natural polymer
- Synthetic polymer
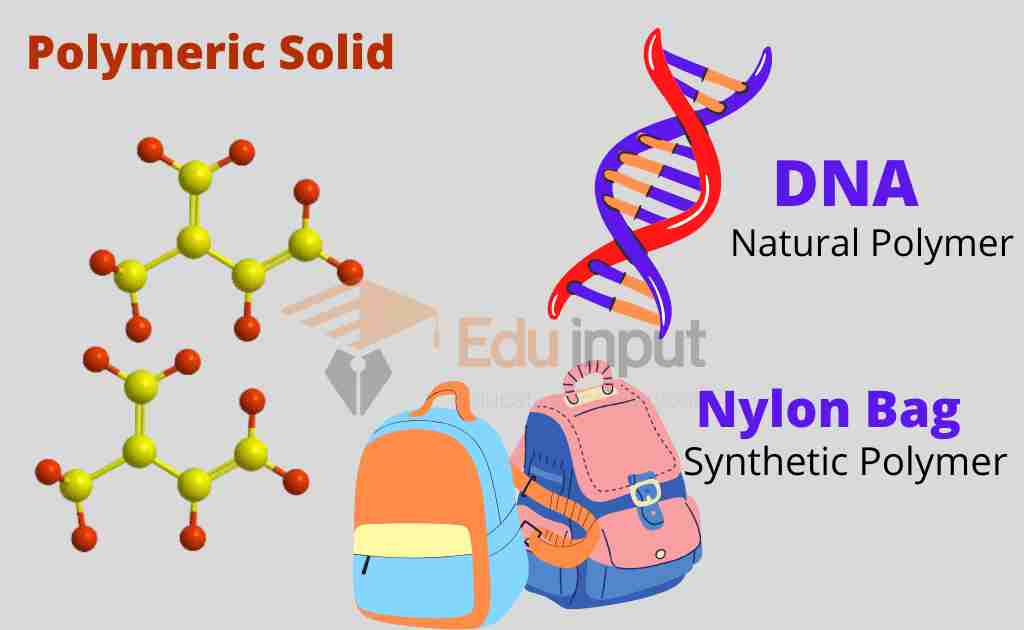
Natural polymers
Natural polymers occur in nature. They are also called biopolymers. DNA, silk, and wool are biopolymers.
Synthetic polymers
Synthetic polymers are produced in different types of reactions. Synthetic polymers include nylon, polyethylene, polyester, Teflon, and epoxy
They form the largest group of naturally occurring and synthetic materials.
Plastic and synthetic rubbers are often called ‘polymers’ because they are formed by polymerization reactions in which relatively simple molecules are chemically combined into massive long-chain molecules or three-dimensional structures.
These molecules have a low specific gravity.
Examples:
Polymers consist of whole or in part of chemical combinations of carbon with oxygen, hydrogen, nitrogen, and other metallic or non-metallic elements.
Polythene, polystyrene, nylon, etc, are examples of polymers.
Natural rubber is compressed in the pure state entirely of a hydrocarbon with the formula (C5H6)n.
Related FAQs
What is Crystal lattice?
A crystalline solid consists of a three-dimensional pattern that repeats itself over and over again.
What is Unit Cell?
The smallest three-dimensional structures are called unit cells.





Leave a Reply