Electrical Properties of Solids | Energy Band Theory
The fundamental electrical property of solids is their ability to conduct electric current. The electrical behavior of different materials is different.
Electrical properties of solids
Classification of solids is based on the bonds that hold the atoms or molecules together. The electrical properties of solids are their ability to conduct electricity.
Solids are classified into three types on the basis of their electrical conductivity.
Conductors
Those materials which conduct electricity easily are called conductors e.g. copper, silver, etc.
Their conductivities are of the order of 107 (Ωm)-1.
Insulators
Those materials which do not conduct electricity are called insulators.
For example wood, diamond, etc with conductivities ranging between 10-10 to 10-2 (Ωm)-1.
Semiconductors
These materials are not good as conductors and not bad as insulators are called semiconductors. For example silicon etc. Their conductivities range from 10-6 to 10-4 (Ωm)-1.
The conventional free electrons theory based on the Bohr model of electron distribution failed to explain completely the electrical behavior of these three solids.
Band theory of solids is used for this purpose which is based on the wave mechanical model.
Energy band theory
Electrons in a single isolated atom have discrete energies and are bound to their nuclei. In solids, when a large number of atoms, say N, comes close enough to each other to form a solid, each energy level of an atom splits up into N sub-levels, called states.
This action is due to the action force exerted due to other atoms in the solid. These energy states are discrete but so close to each other that they look like continuous energy bands.
Forbidden Energy Band
There is a range of energy states which cannot be occupied by electrons. These are called forbidden energy states and their range is called the forbidden energy gap.
Valence Band
The electrons in the outermost shell are called valence electrons and the energy band formed by these electrons is called the valence band. This band may be either completely filled or partially filled but cannot be empty.
Conduction Band
The band above the valence band is called the conduction band. In this band, the electrons move freely and conduct electric current. The electrons in this band are called conduction electrons or free electrons. This band may be partially filled or empty.
Other Band
The bands below the conduction and valence bands are normally completely filled and they do not have any role in the electrical conduction process.
On the basis of the band theory of solids the conductors, insulators, and semiconductors are classified as under.
Insulators:
In insulators, the electrons are tightly bound in their valence shells and they are not free to move.
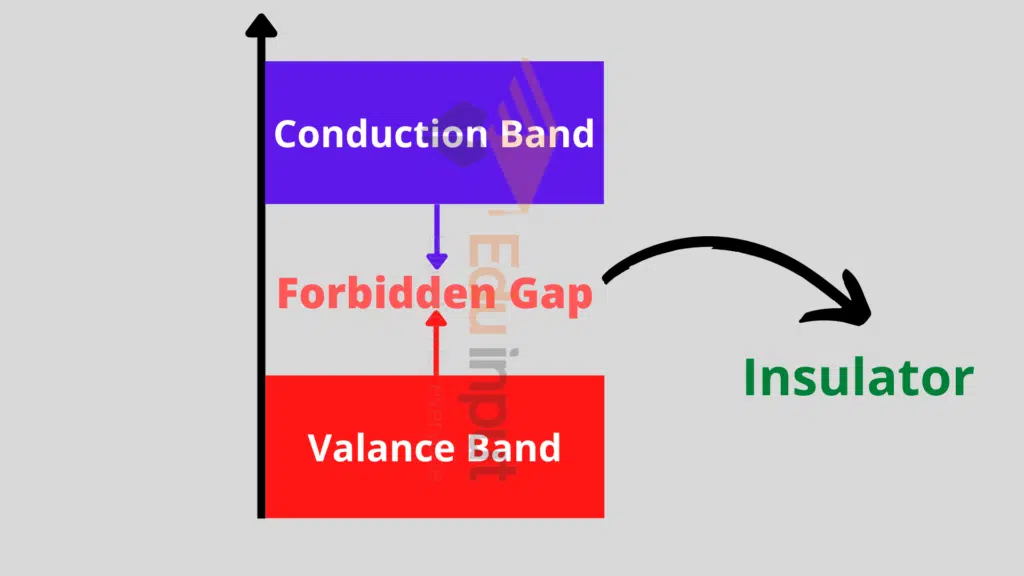
In these materials, the valence band is completely filled and the conduction band is empty. There is a large energy gap between the conduction and the valence band.
And due to this difference, they cannot move from the valence to the conduction band.
Conductors:
Conductors are those materials that have a large number of free electrons for electrical conduction.
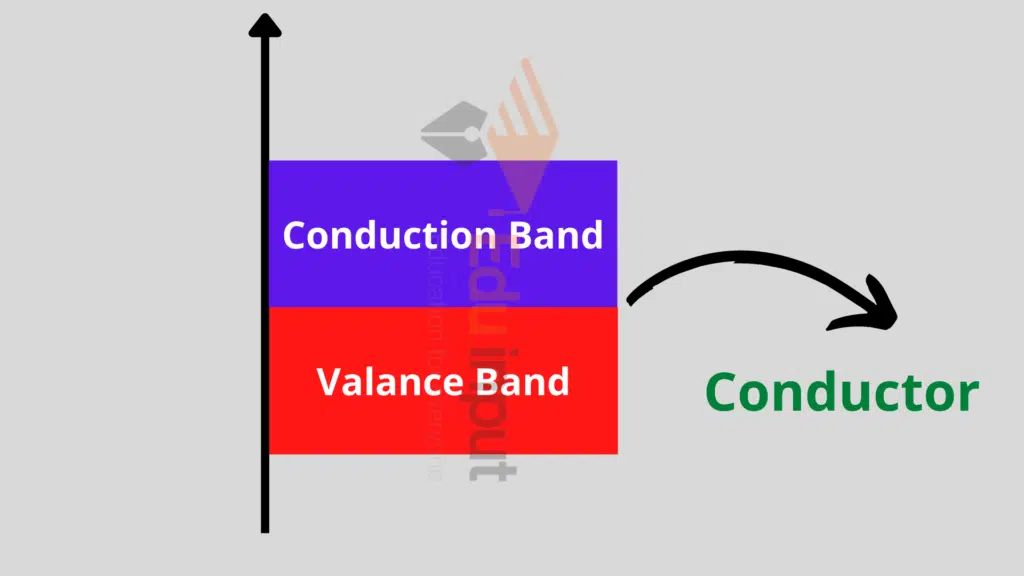
In terms of energy band, the valence and the conduction bands are overlapped i.e. there is no distinction between these two bands.
Semiconductors:
These are those materials that have a partially filled valence band and a partially filled conduction band. A very narrow energy gap between the two bands exists and is normally of the order of 1eV.
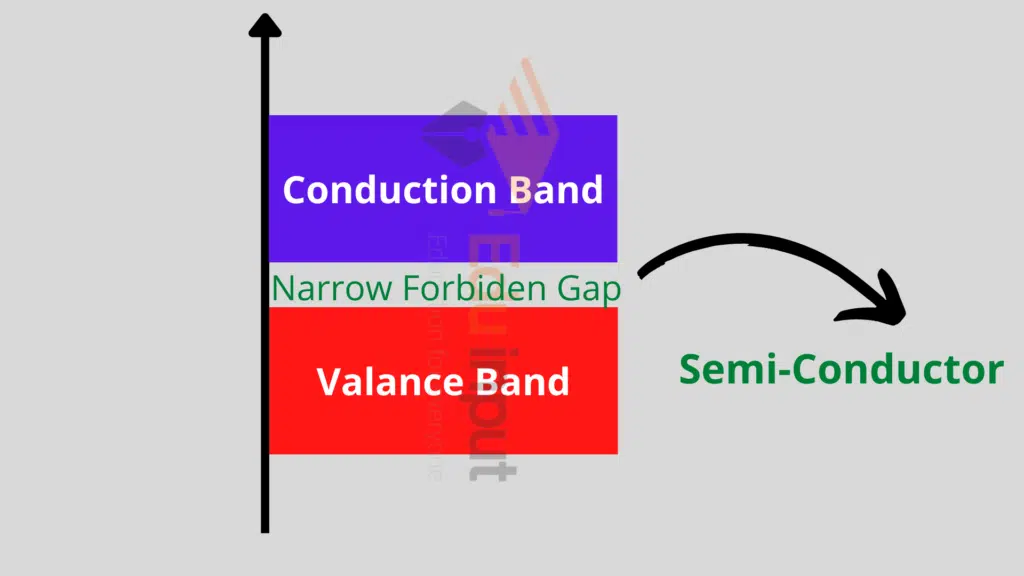
At OK, there are no electrons in the conduction band and the valence band is completely filled. This means that at 0K, the Ge and Si behave like insulators.
With the increase in temperature, few of the electrons gain sufficient energy to move from the valence band to the conduction band.
When electrons move from the valence band to the conduction band, they leave behind a vacancy in the valence band. This vacancy is called a hole. The hole has a positive charge.
Related FAQs
What are the 3 electrical properties of solids?
The electrical properties of solids are their ability to conduct electrical current. Various electrical properties are resistivity, Electrical conductivity, temperature coefficient of resistance, dielectric strength, and thermoelectricity.
Do solids have electrical conductivity?
Electrical conductivity is one of the most important properties of solids. On the basis of it, we distinguish among three types of solids: conductors (or metals ), insulators, and semiconductors. The last ones have intermediate conductivity compared to insulators and conductors.
Which solid has the highest electrical conductivity?
Silver has the highest electrical conductivity of all metals. In fact, silver defines conductivity as all other metals are compared against it. On a scale of 0 to 100, silver ranks 100, with copper at 97 and gold at 76.
What type of solids is an electrical insulator?
Ionic solids are non-conductors of electricity in the solid state, as the constituent ions are not free to move (but in a molten state or when dissolved in water the ions are free to move, and hence they conduct electricity)




Leave a Reply