Hassium-Discovery, Properties, And Applications
Hassium is a synthetic element with the atomic number 108 and the symbol Hs. It is a highly unstable element that has only been produced in small quantities in laboratories. Hassium belongs to the group of transuranium elements and is one of the heaviest elements in existence.

| Property | Value |
| Name | Hassium |
| Symbol | Hs |
| Atomic number | 108 |
| Relative atomic mass (Ar) | (longest lived isotope) |
| Standard state | Presumably a solid at 298 K |
| Appearance | Unknown, probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| Group in periodic table | 8 |
| Group name | (none) |
| Period in periodic table | 7 |
| Block in periodic table | d |
| Shell structure | 2.8.18.32.32.14.2 |
| CAS Registry | 54037-57-9 |
Discovery
Hassium was first synthesized in 1984 by a team of German scientists led by Peter Armbruster and Gottfried Münzenberg. They bombarded lead-208 with iron-58 ions to create hassium-265, which decayed quickly into other elements.
Physical Properties
Hassium is a highly unstable element that is not found naturally on Earth. It has a very short half-life of only a few seconds, which makes it very difficult to study. Hassium is a dense metal that is likely to be silvery or gray in color. It is also likely to be highly radioactive and potentially hazardous to human health.
Chemical Properties
Hassium is a highly reactive element that has only been produced in small quantities in laboratories. It is likely to react with many other elements, forming various chemical compounds. Hassium has been shown to form a volatile chloride compound in laboratory experiments.
Electronic Configuration of Hassium
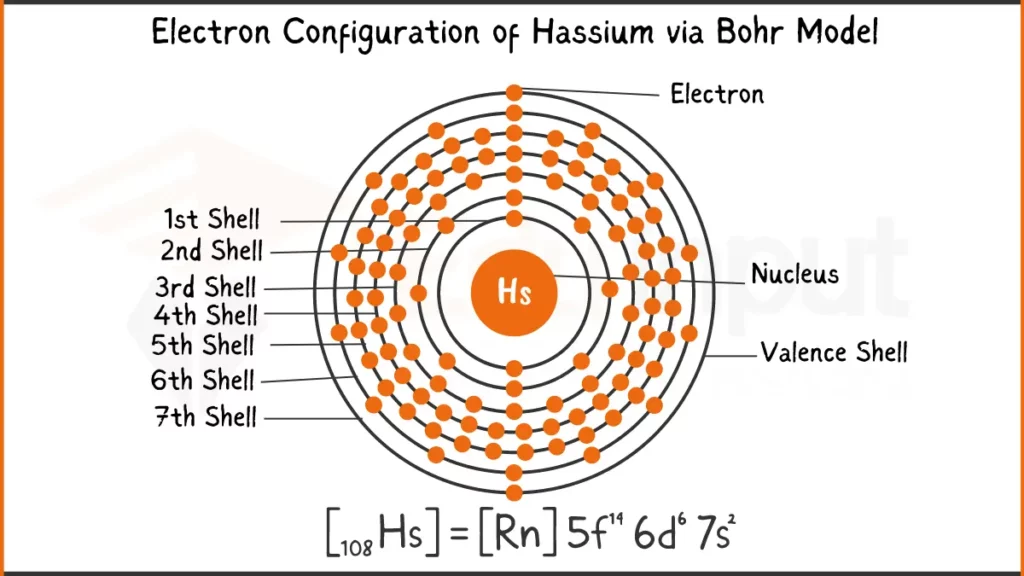
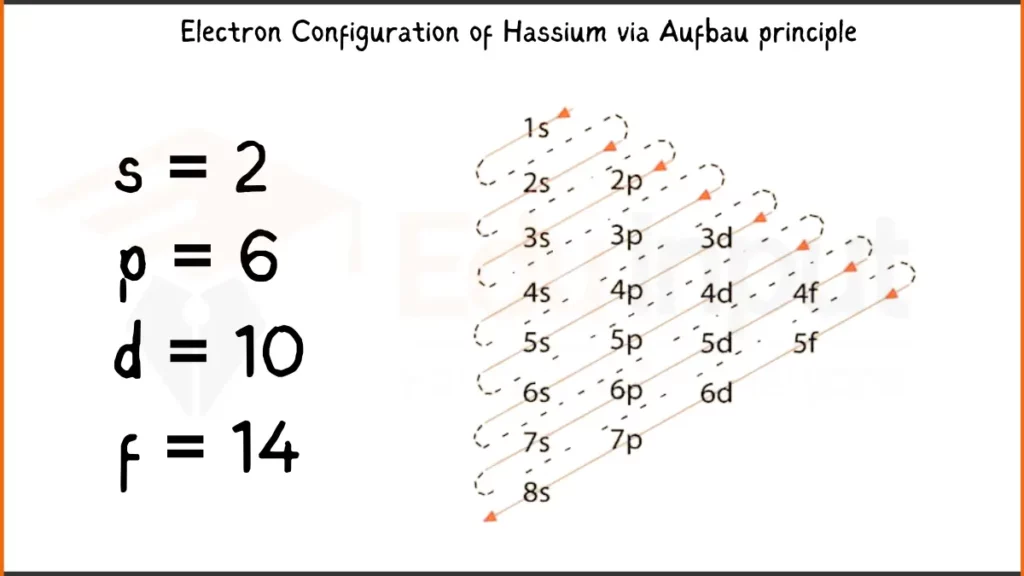
Hassium (Hs) has 108 electrons. Its electron configuration is [Rn] 5f¹⁴ 6d⁶ 7s², which means it has 6 filled shells represented by [Rn] (Radon), 14 electrons in the 5f subshell, 6 electrons in the 6d subshell, and 2 electrons in the 7s subshell. The valence electrons of Hassium are in the 6d subshell, which has an unusual configuration of six electrons (6d⁶).
Electronic Configuration of Hassium via Bohr Model
Electronic Configuration of Hassium via Aufbau Principle
Facts
- Hassium is a synthetic and highly unstable element that has only been produced in small quantities in laboratories.
- It was first synthesized in 1984 by a team of German scientists led by Peter Armbruster and Gottfried Münzenberg.
- Hassium has a very short half-life of only a few seconds, which makes it difficult to study.
Applications
As hassium is a highly unstable and radioactive element that has only been produced in small quantities in laboratories, it has no practical applications in the real world. However, it is of great scientific interest to researchers who study the properties and behavior of heavy and unstable elements.
Hassium is a synthetic and highly unstable element that has only been produced in small quantities in laboratories. It has no practical applications in the real world, but it is of great scientific interest to researchers studying the properties and behavior of heavy and unstable elements.





Leave a Reply