Arsenic-Discovery, Properties, And Applications
Arsenic is a chemical element with the symbol As and atomic number 33. It is a metalloid, which means it has properties of both metals and nonmetals. It is commonly found in minerals such as arsenopyrite, realgar, and orpiment.
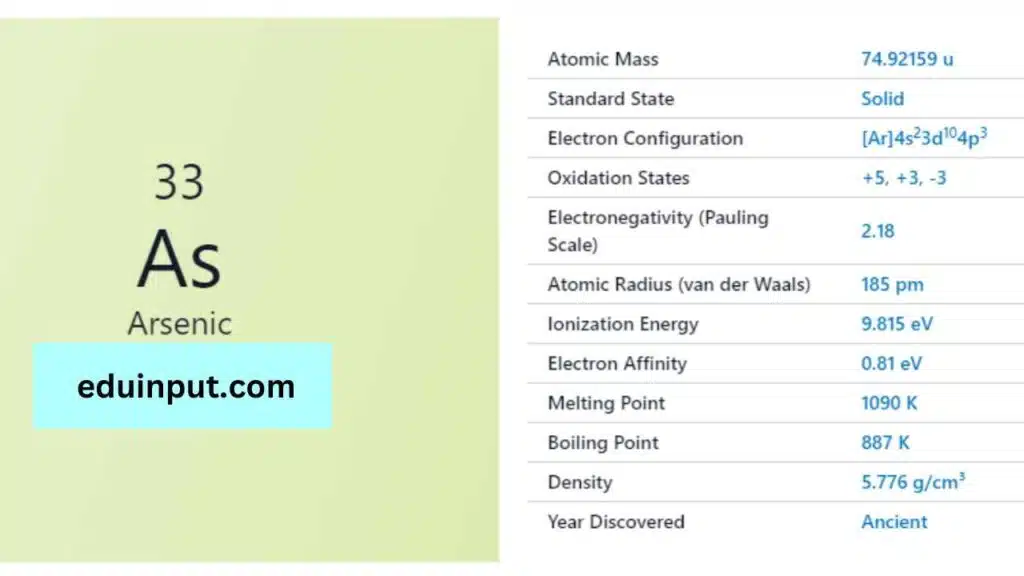
| Property | Value |
| Name | Arsenic |
| Symbol | As |
| Atomic number | 33 |
| Relative atomic mass (Ar) | 74.921595 |
| Standard state | Solid at 298 K |
| Appearance | Metallic grey |
| Classification | Semi-metallic |
| Period in the periodic table | 15 |
| Group name | Pnictogen |
| Period in periodic table | 4 |
| Block in periodic table | p |
| Shell structure | 2.8.18.5 |
| CAS Registry | 7440-38-2 |
Discovery
Arsenic has been known since ancient times, with its use as a poison documented in ancient Rome and Greece. It was first identified as an element by Albertus Magnus in the 13th century.
Physical Properties
Arsenic is a brittle, silvery-gray metalloid. It has a density of 5.73 g/cm3 and a melting point of 817 °C. It can exist in several allotropes, including yellow, black, and gray arsenic.
Chemical Properties
Arsenic is a member of group 15 in the periodic table, also known as the nitrogen group. It readily forms covalent bonds with other elements and is a powerful reducing agent. It is also known to form alloys with many metals, including copper and lead.
Facts
- Arsenic is highly toxic and exposure to even small amounts can cause serious health problems.
- In the past, arsenic was used in a variety of products, including pesticides, wood preservatives, and cosmetics.
- The use of arsenic-based pesticides has been banned in many countries due to their toxicity.
- Arsenic is used in the production of semiconductors and in some medical treatments.
Applications
- Arsenic is used in the production of semiconductors, particularly in the manufacture of gallium arsenide-based devices.
- It is also used in the production of lead alloys, such as those used in car batteries.
- Arsenic trioxide has been used as a medical treatment for leukemia, although its use is controversial due to its toxicity.
- In the past, arsenic was used as a wood preservative and pesticide, but these uses have largely been discontinued due to its toxicity.





Leave a Reply