Copernicium-Discovery, Properties, And Applications
Copernicium is a synthetic chemical element with the symbol Cn and atomic number 112. It is a highly radioactive element and can only be created in a laboratory.
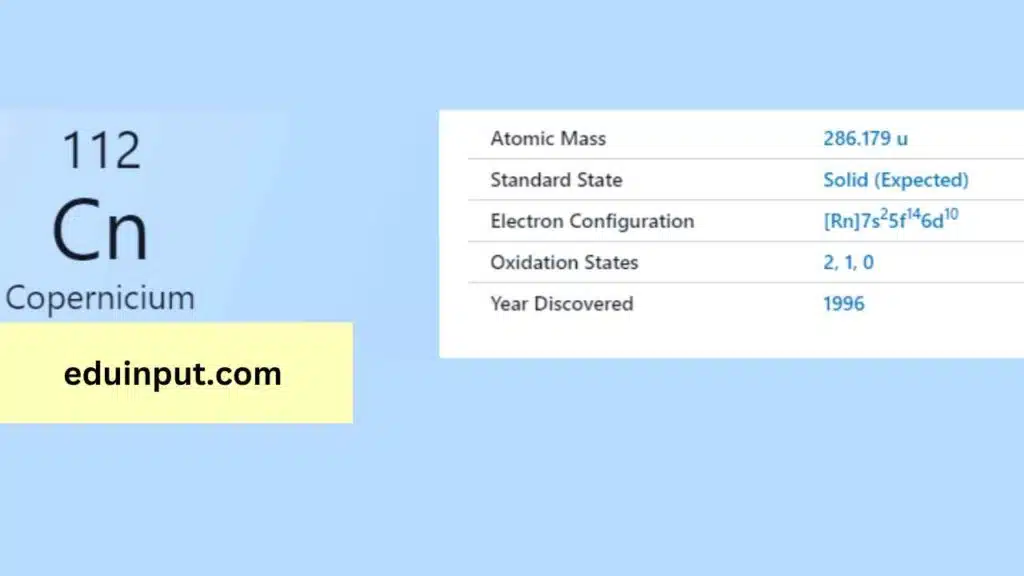
| Property | Value |
| Name | Copernicium |
| Symbol | Cn |
| Atomic number | 112 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Presumably a liquid at 298 K |
| Appearance | Unknown, probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| Period in the periodic table | 12 |
| Group name | (none) |
| Block in the periodic table | 7 |
| Block in periodic table | d |
| Shell structure | 2.8.18.32.32.18.2 |
| CAS Registry | 54084-26-3 |
Discovery
Copernicium was first synthesized in 1996 by a team of German and Russian scientists at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany. They bombarded lead-208 with zinc-70 ions to produce copernicium-277, which then decayed to form copernicium-277.
Physical Properties
Copernicium is a highly unstable element and does not exist naturally on Earth. Its most stable isotope, copernicium-285, has a half-life of only 29 seconds. It is a member of the group 12 elements, which includes zinc, cadmium, and mercury. Copernicium is a dense, metallic element with a silver-white color.
Chemical Properties
As an extremely heavy element, copernicium is expected to behave similarly to its lighter homologs zinc, cadmium, and mercury. However, due to its extreme instability, copernicium has not been studied extensively and its chemical properties are not well known.
Facts
- Copernicium is named after the Polish astronomer Nicolaus Copernicus.
- Its atomic symbol, Cn, is derived from Copernicus’s name.
- Copernicium is one of the heaviest elements ever created.
- It has no known biological role and is highly toxic.
Applications
As a highly radioactive and unstable element, copernicium has no practical applications. Its study is mainly of scientific interest in the field of nuclear physics and chemistry.







Leave a Reply