Zinc-Discovery, Properties, And Applications
Zinc is a chemical element that has the symbol Zn and atomic number 30. It is a slightly brittle, bluish-white metal that is found in minerals, soil, air, and water. Zinc is an essential nutrient for living organisms and plays a vital role in various biological processes.
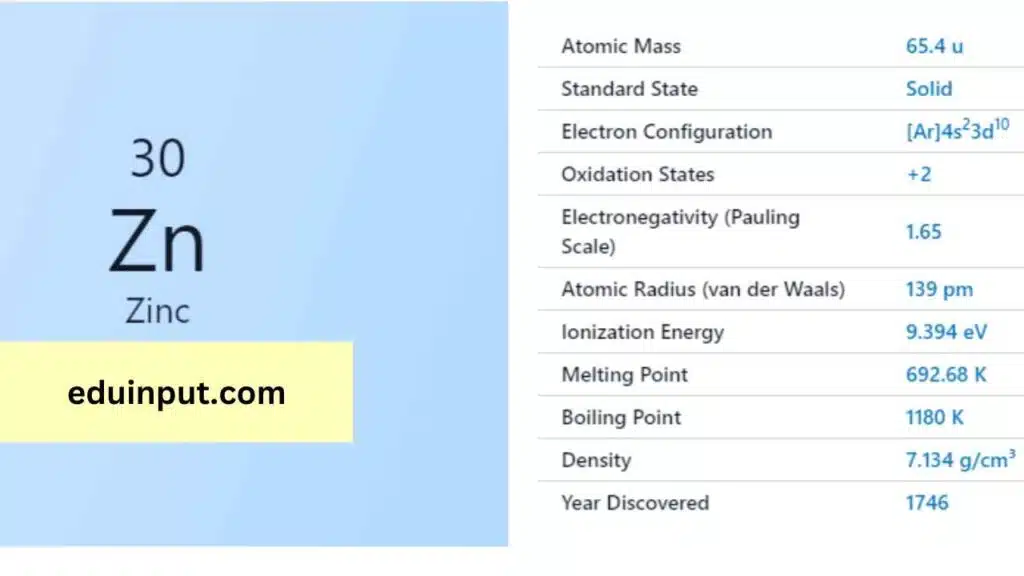
| Property | Value |
| Name | Zinc |
| Symbol | Zn |
| Atomic number | 30 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Bluish pale grey |
| Classification | Metallic |
| Block in the periodic table | 12 |
| Group name | (none) |
| Period in periodic table | 4 |
| Period in the periodic table | d |
| Shell structure | 2.8.18.2 |
| CAS Registry | 7440-66-6 |
Discovery
Zinc has been used by humans for thousands of years, but its discovery as an element is relatively recent. In the early 16th century, the German alchemist Andreas Libavius described a substance called “zincum” in his writings. The first recorded isolation of metallic zinc was done by Andreas Marggraf, a German chemist, in 1746.
Physical Properties
Zinc is a relatively soft metal, with a Mohs hardness of 2.5. It has a melting point of 419.5°C and a boiling point of 907°C. Zinc is a good conductor of electricity and has a low thermal conductivity. It is also highly ductile and malleable, meaning it can be easily shaped into different forms.
Chemical Properties
Zinc is a reactive metal that readily forms compounds with other elements. It has a standard electrode potential of -0.76V, which means it can easily give up electrons to form positive ions. Zinc reacts with acids to produce hydrogen gas and with alkalis to form zincates. It also reacts with oxygen in the air to form a protective layer of zinc oxide, which prevents further corrosion.
Facts
- Zinc is the 24th most abundant element on Earth and is found in many minerals, including sphalerite, smithsonite, and hemimorphite.
- The largest producers of zinc are China, Peru, and Australia.
- Zinc is commonly used as a protective coating for iron and steel, as it helps prevent corrosion.
- The human body requires zinc for various biological processes, including immune function, wound healing, and DNA synthesis.
Applications
Zinc has many industrial applications, including:
- Galvanizing: Zinc is used to coat iron and steel to protect them from corrosion.
- Die-casting: Zinc is used to create intricate parts for machines and vehicles.
- Batteries: Zinc is used in alkaline batteries as a cathode material.
- Zinc oxide: Zinc oxide is used in many products, including paint, rubber, ceramics, and cosmetics.
In addition to its industrial uses, zinc is also an essential nutrient for living organisms. It is found in many foods, including meat, seafood, and legumes, and is often added to multivitamin supplements. Zinc deficiency can cause a range of health problems, including impaired immune function, delayed wound healing, and growth retardation.







Leave a Reply