What are Cofactor and Coenzyme? – What Do They Do?
The metal ions and non-protein parts of an enzyme are called the cofactor. Which are essential for the functioning of the enzymes inside the cell. But some enzymes consist for the proper solely of proteins.
Some of the cofactors like ATP are manufactured inside the body. A cofactor may be an organic compound like vitamins. it is considered a helper molecule for biochemical reactions.
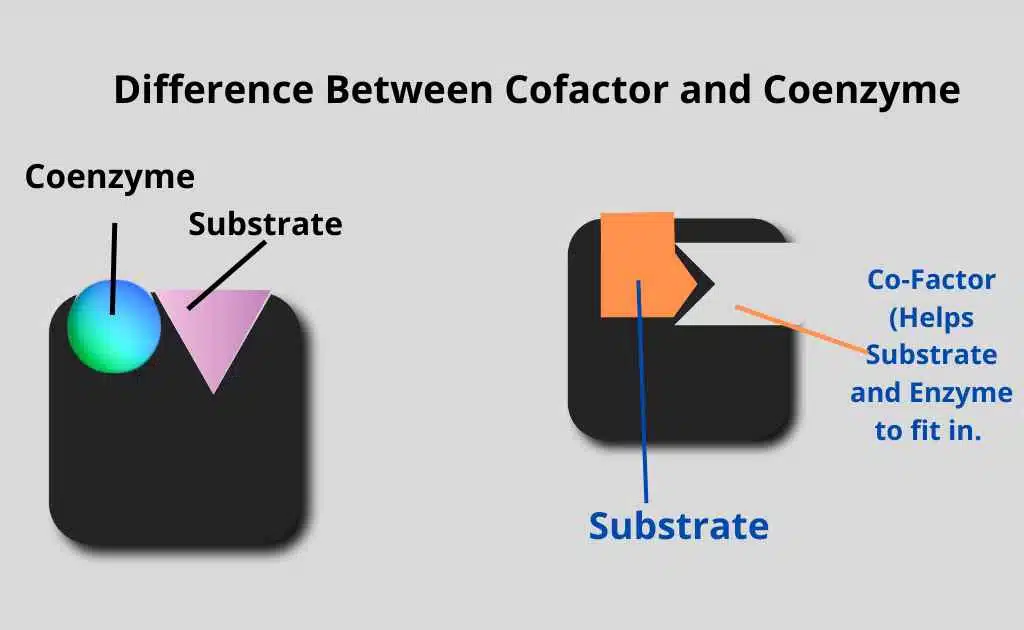
These metal ions are loosely attached to the enzymes. These metal ions activate a nonfunctioning active site. As a cofactor attaches to an enzyme or protein, it changes its shape and makes them fold or bind a little bit. It allows the formation of enzyme-substrate complex or enzyme-substrate attachment.
The cofactors of enzymes participate in the temporary bond formation, which forms between the enzyme and its substrate during enzyme-substrate complex formation.

Examples of Cofactors
Examples of coenzymes are
- NAD (Nicotinamide Adenine Dinucleotide)
- NADP (Nicotinamide Adenine Dinucleotide Phosphate)
- FAD (Flavin Adenine Dinucleotide)
- Calcium Ion – Transglutaminase
- Mangenese Ion – Arginase Enzyme
Types of Cofactors:
There are three types of co-factors:
Activator: The detachable co-factor is known as an activator. These co-factors are inorganic ions like Mg. Fe, Cu, Zn, etc.
B) Prosthetic group: The covalently bonded non-protein part cofactor is known as, the prosthetic group.
C) Coenzyme: Non-Protein organic molecules that are involved in the catalytic reaction.
What are Coenzymes? and What do they Do?
Coenzymes are nonprotein, organic molecules that participate in (enzyme) catalytic reactions. They are used to transport electrons from one enzyme to another. These electrons are present in the form of hydrogen atoms. Many vitamins like niacin and riboflavin function as coenzymes. Or they are used to make coenzymes.
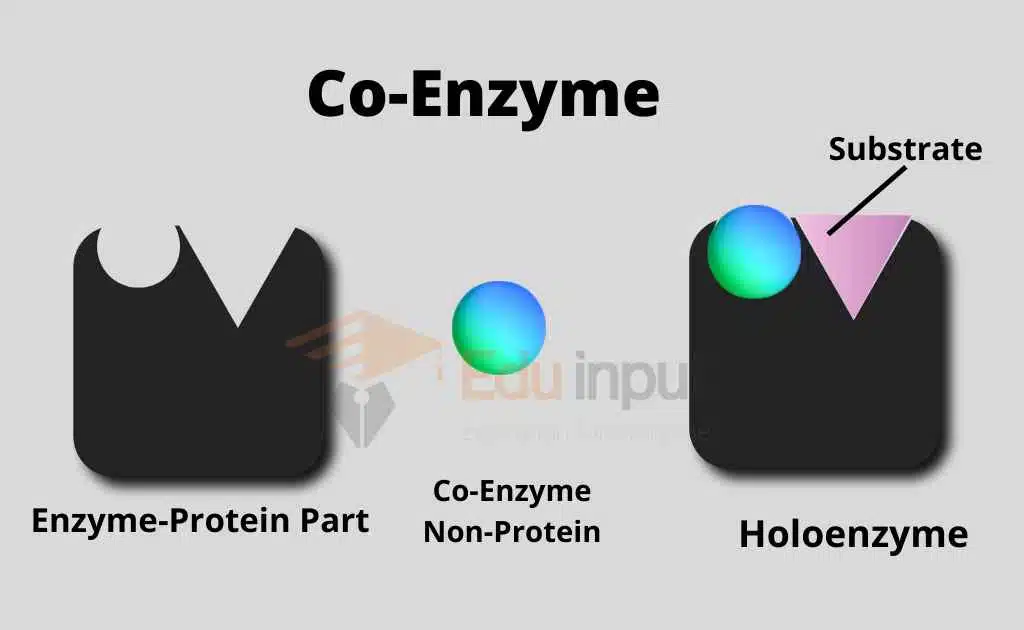
Coenzymes transport energy in the form of hydrogen atoms. Coenzymes are related to vitamins. Vitamins are essential raw materials for the synthesis of coenzymes. Coenzymes can be used again and again. Therefore, a quantity of vitamins is needed.
Examples of Coenzyme
- Vitamin B3 (Niacin)
- Vitamin B12 (Cyanocobalamin)
- Thiamine Pyrophosphate (Vitamin B1)
- Pyridoxal Phosphate (Vitamin B6)
NAD- An Example of Coenzyme
One of the most important coenzymes in the cell is the hydrogen acceptor NAD. It is made from vitamin B. it acquires a hydrogen atom from an enzyme and then reduces to NADH.
The electron of the hydrogen atom contains energy. This energy is carried by the NADH molecule. For example, various foods are oxidized in the cell. The cell removes electrons from the food molecules. It transfers this electron to NAD. It reduces NADH.
Examples of Coenzyme
- Vitamin B3 (Niacin)
- Vitamin B12 (Cyanocobalamin)
- Thiamine Pyrophosphate (Vitamin B1)
- Pyridoxal Phosphate (Vitamin B6)
- Vitamin C (Ascorbic Acid)
Functions Of Cofactors
- They are essential for the proper functioning of enzymes.
- The co-factors act as a bridge between the enzyme and substrate.
- They often directly take part in chemical reactions during catalysis. Sometimes, the cofactors provide a source of chemical energy. This energy is necessary to drive a reaction.






Leave a Reply