Berkelium-Discovery, Properties, And Applications
Berkelium is a rare and radioactive element with the atomic number 97 and symbol Bk. It belongs to the actinide series and is named after the city of Berkeley in California where it was first discovered. The element is highly toxic and has no commercial applications.
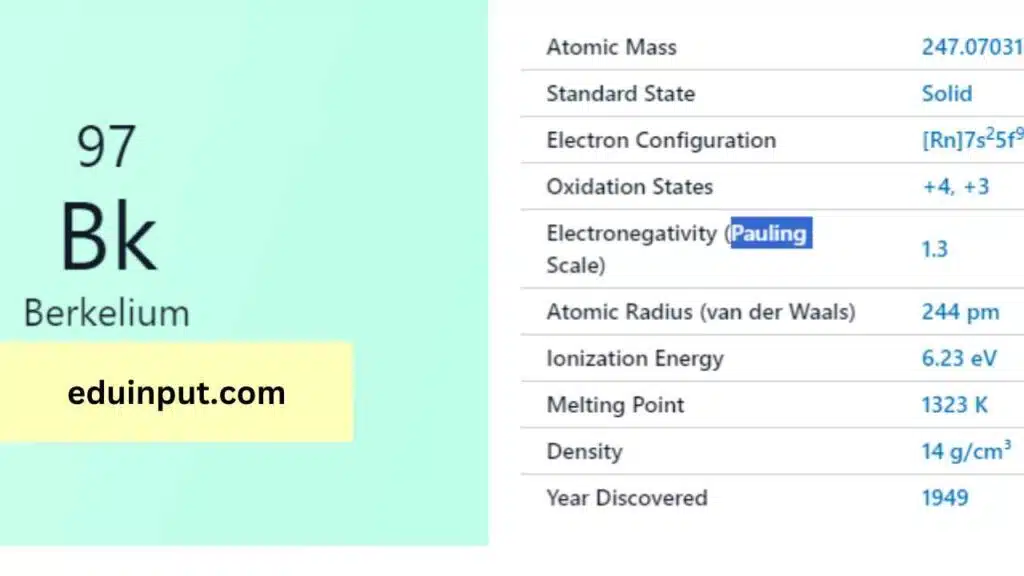
| Property | Value |
| Name | Berkelium |
| Symbol | Bk |
| Atomic number | 97 |
| Relative atomic mass (Ar) | Period in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Unknown, but probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| The group in the periodic table | |
| Group name | Actinoid |
| Block in the periodic table | 7 (actinoid) |
| Group in the periodic table | f |
| Shell structure | 2.8.18.32.27.8.2 |
| CAS Registry | 7440-40-6 |
Discovery
Berkelium was first synthesized in 1949 by Glenn T. Seaborg, Stanley G. Thompson, and Albert Ghiorso by bombarding americium-241 with alpha particles in a cyclotron at the University of California, Berkeley. The element was named after the city of Berkeley and its discovery was kept secret until 1950.
Physical Properties
Berkelium is a silvery-white metal that is radioactive and highly toxic. It has a melting point of 986°C and a boiling point of 2627°C. The element is paramagnetic, which means it is weakly attracted to magnetic fields. It has a density of 14.78 g/cm³, which is similar to that of lead.
Chemical Properties
Berkelium is a highly reactive metal that is easily oxidized in air. It reacts with water, acids, and bases to form various compounds. The element has eight known isotopes, with the most stable being berkelium-247, which has a half-life of 1,380 years.
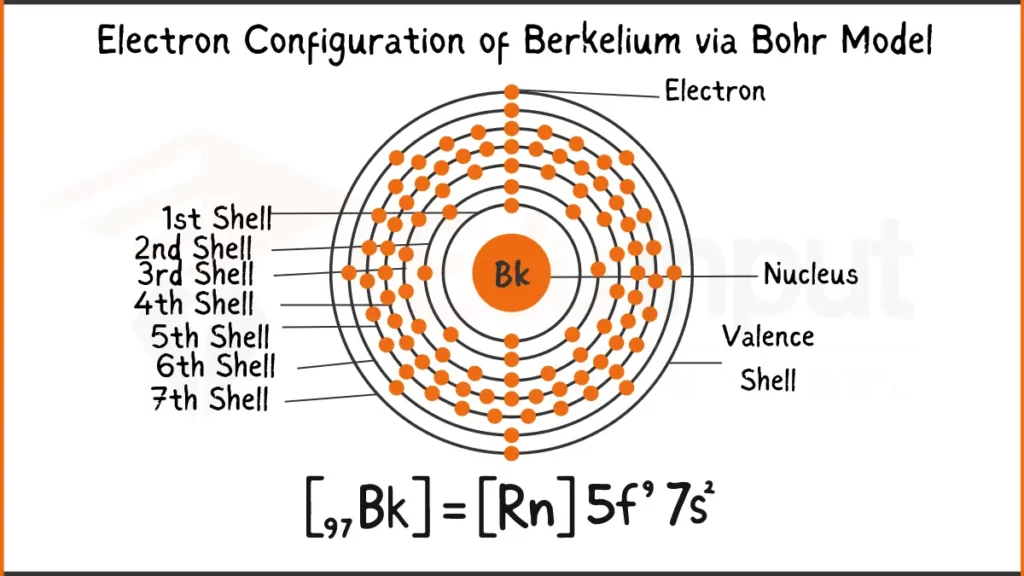
Electron Configuration of Berkelium
Berkelium (Bk) possesses 97 electrons arranged as [Rn]5f⁹7s². This shows it shares the stable inner electron structure of Radon (Rn), with the remaining electrons filling the 5f subshell (9) and the outermost 7s subshell (2).
Electron Configuration of Berkelium Via Bohr Model
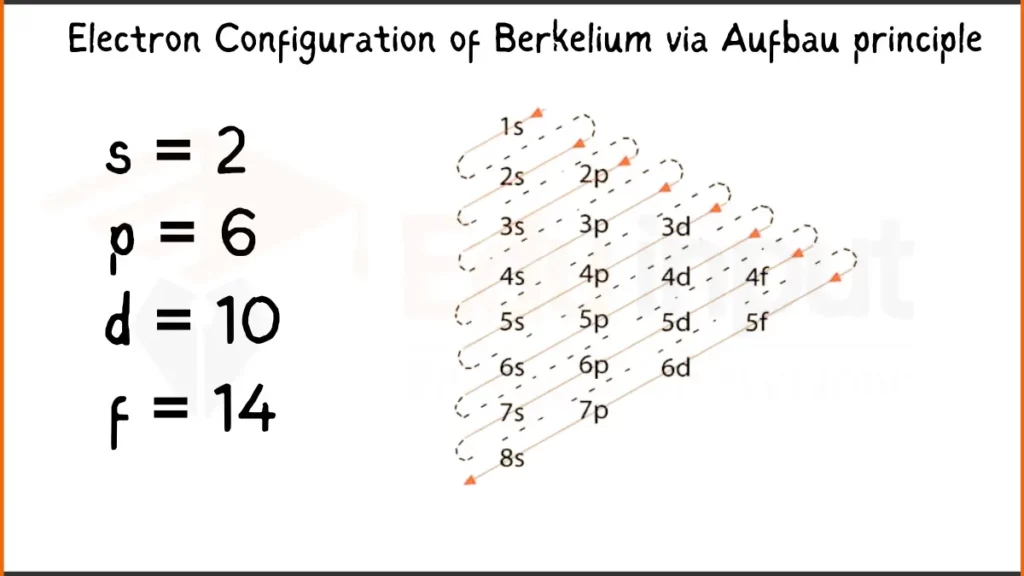
Electron Configuration of Berkelium Via Aufbau Principle
Facts
- Berkelium is used in scientific research to produce heavy isotopes for nuclear medicine and in the study of nuclear physics.
- The element is highly toxic and poses a danger to humans and the environment.
- Berkelium has no commercial applications and is only used in scientific research.
Applications
Berkelium has no commercial applications. It is used in scientific research to produce heavy isotopes for nuclear medicine and in the study of nuclear physics. Its toxicity and radioactive properties make it unsuitable for use in consumer products.






Leave a Reply