Bond Energy, definition, types, factors and applications
Definition
The average amount of energy required to break all bonds of a particular type in one mole of the substance is called bond energy.

What is bond energy?
Bond energy is a fundamental concept in chemistry that plays a crucial role in determining the stability and reactivity of molecules. It refers to the amount of energy required to break a chemical bond and is a key factor in understanding the behavior of atoms and molecules. Bond energy is a measure of the strength of a bond between two atoms.
Bond energy is a central concept in chemistry, influencing the properties and behavior of matter at the molecular level. Its applications extend from predicting the stability of molecules to guiding the design of innovative materials, making it a key focus in the study and application of chemistry.
How is bond energy measured?
Bond energy is typically measured in kilojoules per mole (kJ/mol). Experimental techniques, such as spectroscopy and calorimetry, are used to determine the energy changes associated with breaking and forming bonds. The higher the bond energy, the stronger the bond.
Why is bond energy important?
Understanding bond energy is essential for several reasons. It provides insights into the stability of molecules, influences the design of new materials, and plays a pivotal role in predicting and interpreting chemical reactions. Bond energy is a crucial parameter in the study of chemical kinetics and thermodynamics.
Types of Bond Energy
Covalent Bonds
Covalent bonds involve the sharing of electrons between two atoms.
The strength of a covalent bond is determined by factors such as the number of shared electrons and the distance between the nuclei.
Ionic Bonds
Ionic bonds result from the transfer of electrons from one atom to another.
The electrostatic attraction between oppositely charged ions contributes to the bond energy.
Metallic Bonds
Metallic bonds occur between metal atoms.
The delocalized electrons in metallic bonds contribute to the unique properties of metals, such as conductivity and malleability.
Hydrogen Bonds
Hydrogen bonds form between a hydrogen atom and a highly electronegative atom (usually oxygen, nitrogen, or fluorine).
While weaker than covalent bonds, hydrogen bonds are crucial in determining the properties of substances like water and biological molecules.
Factors Affecting Bond Energy
Electronegativity
The electronegativity difference between atoms influences bond polarity and, consequently, bond strength.
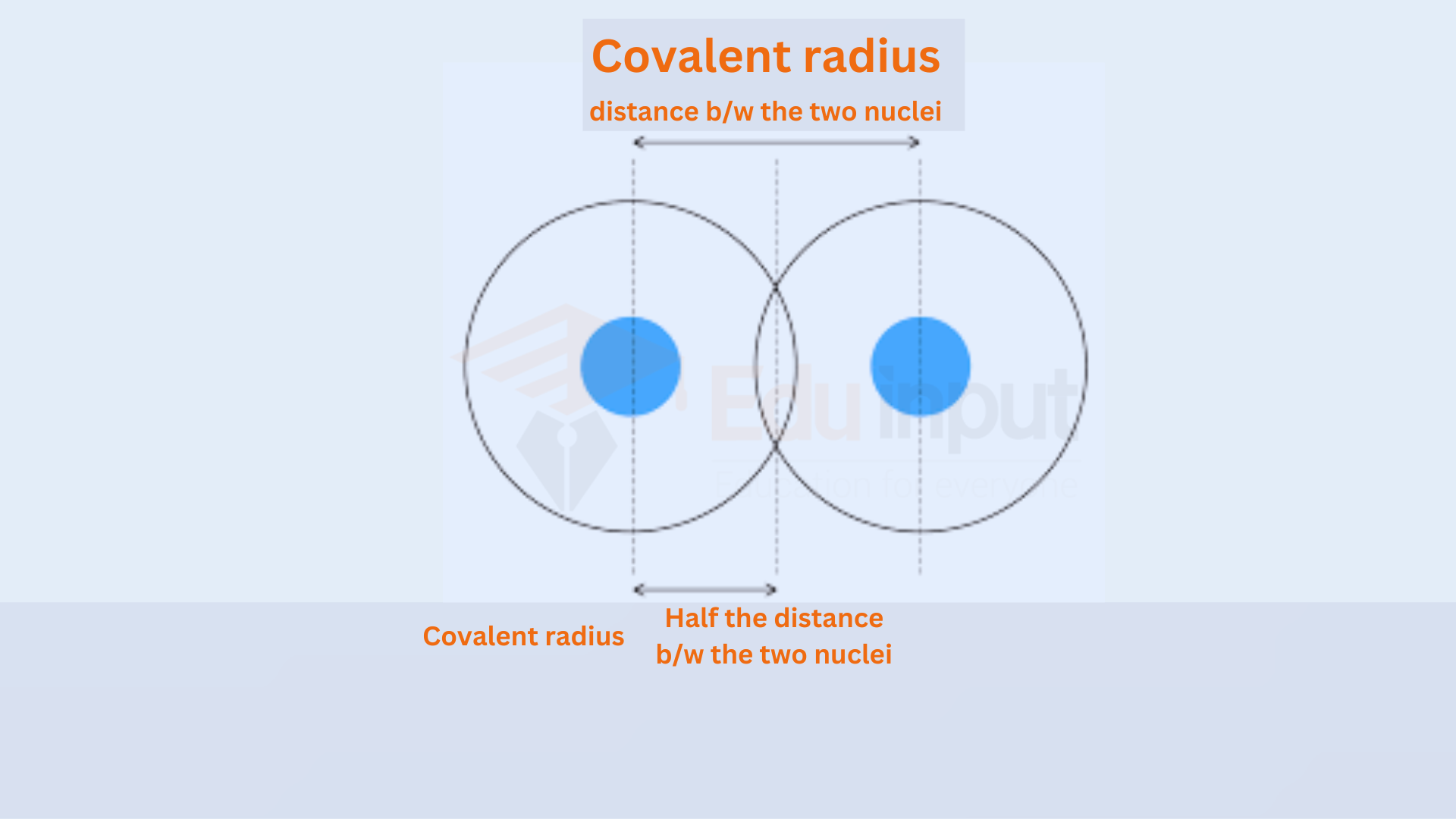
Bond Length
Generally, shorter bonds are stronger. As atoms come closer, the overlap of electron orbitals increases, leading to a stronger bond.
Bond Order
Bond order, indicating the number of bonds between two atoms, affects bond strength. Double and triple bonds are generally stronger than single bonds.
Resonance
Resonance structures influence bond stability. Molecules with resonance exhibit a delocalization of electrons, affecting the overall bond energy.
Hybridization
Hybridization of atomic orbitals influences the arrangement of electrons and, consequently, bond strength.
Applications of Bond Energy
Predicting the Stability of Molecules
High bond energy indicates a stable molecule. Understanding bond strengths helps predict the stability of different molecular structures.
Designing New Materials
Engineers and materials scientists use knowledge of bond energy to design materials with specific properties, such as strength, flexibility, and conductivity.
Understanding Chemical Reactions
Bond energy changes during chemical reactions provide insights into reaction mechanisms, energetics, and whether a reaction is exothermic or endothermic.
What is bond energy, and how is it defined?
Bond energy is the amount of energy required to break a chemical bond in a mole of molecules, or alternatively, it is the energy released when a mole of molecules forms bonds. It is typically measured in kilojoules per mole (kJ/mol).
How is bond energy experimentally determined?
Bond energy is determined experimentally through techniques such as calorimetry or spectroscopy. Calorimetry involves measuring the heat changes associated with a reaction, while spectroscopy analyzes the absorption or emission of light during bond formation or cleavage.
What factors influence the strength of a chemical bond and, consequently, bond energy?
Several factors influence bond strength and bond energy, including electronegativity, bond length, bond order, resonance, and hybridization. Electronegative atoms, shorter bond lengths, and higher bond orders generally lead to stronger bonds.
How does bond energy relate to the stability of molecules?
Bond energy is directly related to the stability of molecules. Higher bond energies indicate stronger bonds and more stable molecules. Stable molecules are less likely to undergo spontaneous chemical reactions.
What are the practical applications of understanding bond energy?
Understanding bond energy has various practical applications. It is crucial in predicting the stability of molecules, designing new materials with specific properties, and elucidating the energetics of chemical reactions. Knowledge of bond energy also contributes to fields such as chemical engineering and materials science.

 written by
written by 



Leave a Reply