Californium-Properties And Applications
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. It is a member of the actinide series of elements and is one of the rarest elements on Earth.
Californium was first synthesized in 1950 at the University of California, Berkeley, by bombarding curium with alpha particles.

| Property | Value |
| Name | Californium |
| Symbol | Cf |
| Atomic number | 98 |
| Relative atomic mass (Ar) | (longest-lived isotope) |
| Standard state | Solid at 298 K |
| Appearance | Unknown, but probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| Group in periodic table | |
| Group name | Actinoid |
| Period in periodic table | 7 (actinoid) |
| Block in periodic table | f |
| Shell structure | 2.8.18.32.28.8.2 |
| CAS Registry | 7440-71-3 |
Physical Properties
Californium is a silvery-white metal that tarnishes in the air. It is very radioactive and produces heat as it decays. It has a melting point of about 900 °C (1,650 °F) and a boiling point of about 1,470 °C (2,680 °F).
Chemical Properties
Californium is a highly reactive element and easily reacts with oxygen, halogens, and other non-metals. It forms various chemical compounds, such as the oxide, halides, and sulfate.
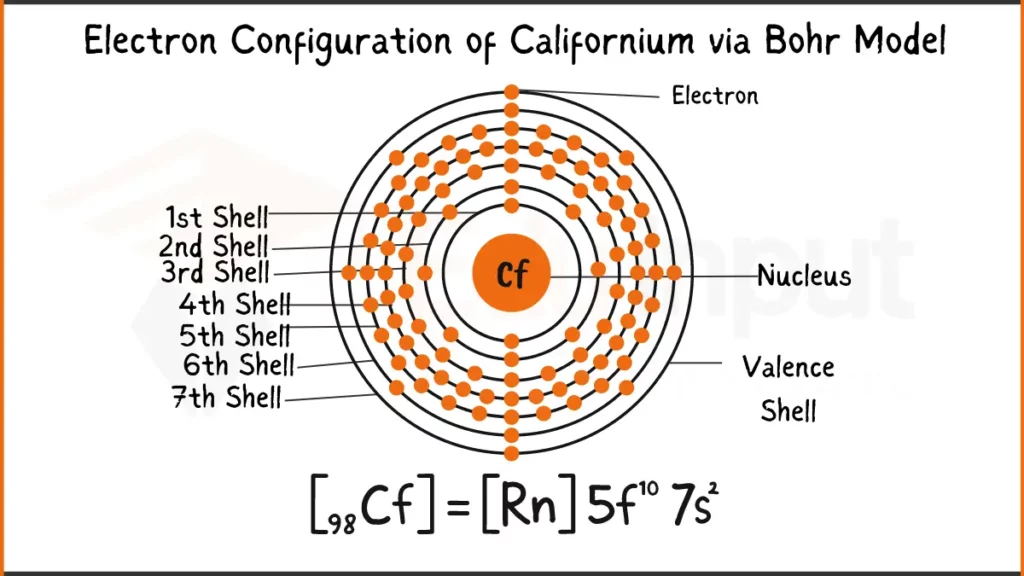
Electron Configuration of Californium
Californium (Cf) has 98 electrons configured as [Rn]5f¹⁰7s². This shows the stable inner electron structure of Radon (Rn), with the remaining electrons filling the 5f subshell (10) and the outermost 7s subshell (2).
Electron Configuration of Californium Via Bohr Model
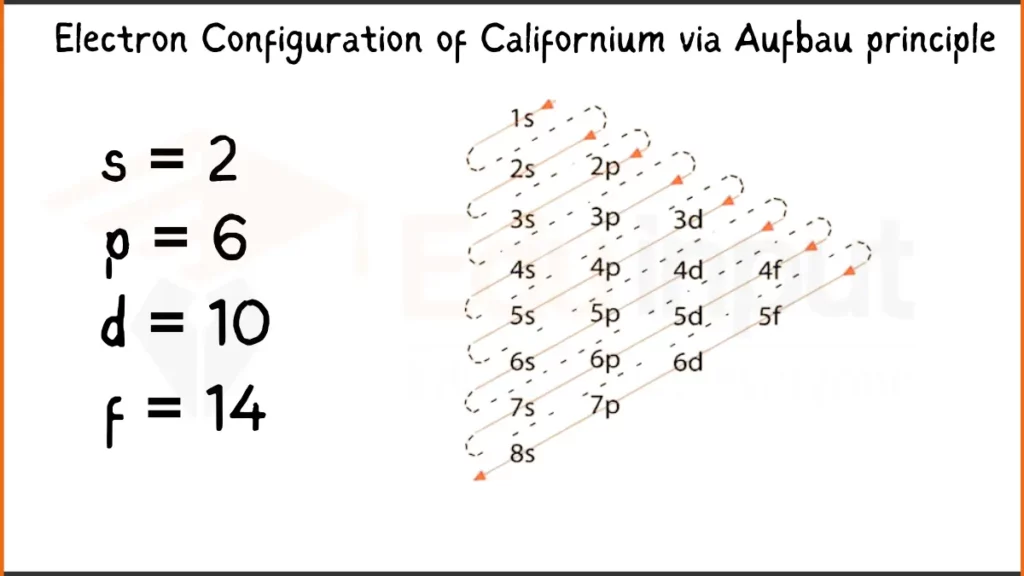
Electron Configuration of Californium Via Aufbau Principle
Facts
Californium is primarily used as a neutron source in nuclear reactors and as a target for the production of heavier elements.
-It is also used in cancer treatment as a radiation source.
-Californium-252 is the most stable isotope of californium and has a half-life of 2.64 years.
-The estimated cost of producing one gram of californium is around 10 million US dollars.
Applications
Californium-252 is used in neutron radiography, which is a non-destructive testing method used to inspect metal parts and structures for defects. It is also used in neutron activation analysis, a technique used to determine the composition of materials. Californium-252 is used in oil well logging to measure the oil and water content of wells.
Californium is a rare and highly radioactive element that has a wide range of applications in nuclear reactors, cancer treatment, and non-destructive testing. Despite its high cost, it is an important element in various scientific and industrial applications.






Leave a Reply