Examples Of Chemical Compounds
Chemical compounds are substances composed of multiple atoms from different elements, bonded together in specific proportions. For example, Calcium carbonate (CaCO₃), a common compound found in limestone, and acetone (C₃H₆O), used as a solvent, are both important in various industries. Glycerol (C₃H₈O₃) is used in pharmaceuticals, while sodium carbonate (Na₂CO₃), commonly known as soda ash, is used in manufacturing glass.
Hydrochloric acid (HCl) is a strong acid in laboratory settings, and aspirin (C₉H₈O₄) is widely used as an anti-inflammatory. Magnesium hydroxide (Mg(OH)₂) is used as an antacid, while nitric acid (HNO₃) plays a vital role in fertilizers. Other examples include potassium permanganate (KMnO₄), a strong oxidizing agent, and calcium chloride (CaCl₂), used for de-icing roads. Each of these compounds has a unique chemical formula, highlighting the diversity of chemical substances, whether organic or inorganic, and their roles in everyday applications.
| Compound Name | Chemical Formula |
|---|---|
| Calcium Carbonate | CaCO₃ |
| Acetone | C₃H₆O |
| Glycerol | C₃H₈O₃ |
| Sodium Carbonate | Na₂CO₃ |
| Hydrochloric Acid | HCl |
| Aspirin | C₉H₈O₄ |
| Magnesium Hydroxide | Mg(OH)₂ |
| Nitric Acid | HNO₃ |
| Potassium Permanganate | KMnO₄ |
| Calcium Chloride | CaCl₂ |
| Glucose | C₆H₁₂O₆ |
| Acetic Anhydride | C₄H₆O₃ |
| Aluminum Sulfate | Al₂(SO₄)₃ |
| Phenol | C₆H₅OH |
| Chloroform | CHCl₃ |
| Sodium Hypochlorite | NaClO |
| Zinc Oxide | ZnO |
| Magnesium Sulfate | MgSO₄ |
| Urea | CH₄N₂O |
| Benzene | C₆H₆ |
What is chemical compound?
Chemical compound is a substance composed of two or more chemically bonded elements.
Chemical compounds are everywhere in our daily lives. They are formed when two or more elements combine. For example, water (H₂O) is a common compound made of hydrogen and oxygen. Table salt (NaCl) is another example of a chemical compound, which is formed by the combination of sodium and chlorine.
These compounds are essential for cooking, cleaning, and keeping healthy. Learning about chemical formulas and how compounds are made helps us understand the world around us.
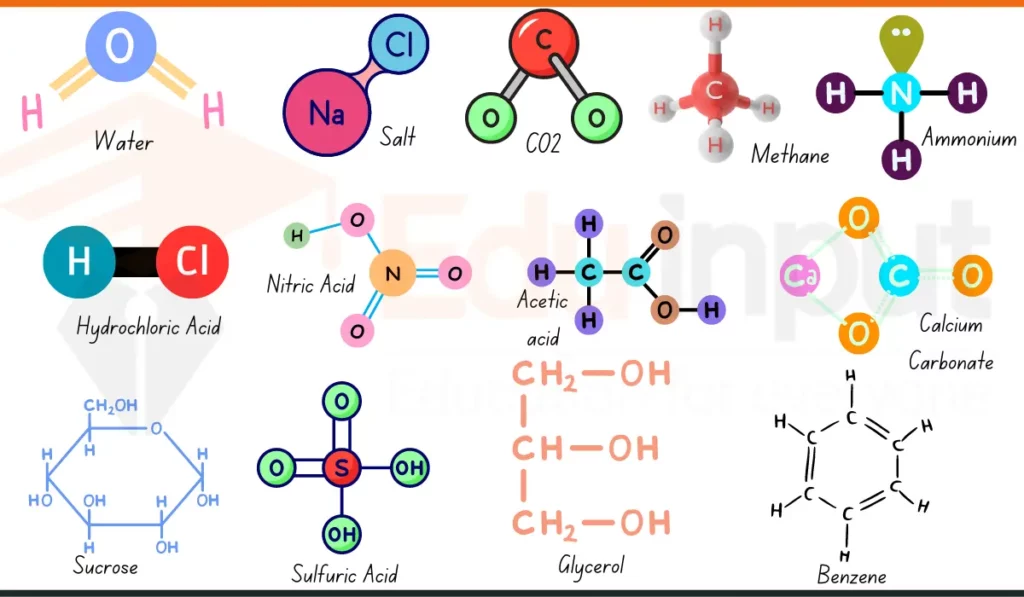
Common Household Compounds
| Compound Name | Chemical Formula | Composition | Use Case |
| Water | H₂O | 2 Hydrogen, 1 Oxygen | Hydration, cooking, cleaning |
| Sodium Chloride | NaCl | 1 Sodium, 1 Chlorine | Seasoning, food preservation |
| Sodium Bicarbonate | NaHCO₃ | 1 Sodium, 1 Hydrogen, 1 Carbon, 3 Oxygen | Baking, cleaning, odor control |
| Acetic Acid | CH₃COOH | 2 Carbon, 4 Hydrogen, 2 Oxygen | Salad dressing, stain removal |
| Isopropyl Alcohol | C₃H₈O | 3 Carbon, 8 Hydrogen, 1 Oxygen | Disinfecting, wound cleaning |
| Glucose | C₆H₁₂O₆ | 6 Carbon, 12 Hydrogen, 6 Oxygen | Body energy, found in foods |
| Methane | CH₄ | 1 Carbon, 4 Hydrogen | Cooking fuel, heating |
| Carbon Dioxide | CO₂ | 1 Carbon, 2 Oxygen | Fizzy drinks, fire extinguishers |
Industrial and Pharmaceutical Compounds
| Compound Name | Chemical Formula | Composition | Use Case |
|---|---|---|---|
| Calcium Carbonate | CaCO₃ | 1 Calcium, 1 Carbon, 3 Oxygen | Cement, paper, antacids |
| Aspirin | C₉H₈O₄ | 9 Carbon, 8 Hydrogen, 4 Oxygen | Pain relief, anti-inflammatory |
| Sodium Carbonate | Na₂CO₃ | 2 Sodium, 1 Carbon, 3 Oxygen | Glass and detergent production |
| Ammonia | NH₃ | 1 Nitrogen, 3 Hydrogen | Fertilizers, household cleaners |
| Sodium Hydroxide | NaOH | 1 Sodium, 1 Oxygen, 1 Hydrogen | Soap making, drain cleaner |
Environmental and Agricultural Compounds
| Compound Name | Chemical Formula | Composition | Use Case |
|---|---|---|---|
| Urea | CH₄N₂O | 1 Carbon, 4 Hydrogen, 2 Nitrogen, 1 Oxygen | Fertilizer, soil nutrient booster |
| Calcium Chloride | CaCl₂ | 1 Calcium, 2 Chlorine | Road de-icing, dust control |
| Nitric Acid | HNO₃ | 1 Hydrogen, 1 Nitrogen, 3 Oxygen | Fertilizer production (ammonium nitrate) |
| Hydrogen Peroxide | H₂O₂ | 2 Hydrogen, 2 Oxygen | Wound cleaning, water purification |
| Sulfur Hexafluoride | SF₆ | 1 Sulfur, 6 Fluorine | Electrical insulation, climate research |
What are 30 Examples Of Chemical Compounds?
Here is the list of chemical compounds, their common names, and their formulas:
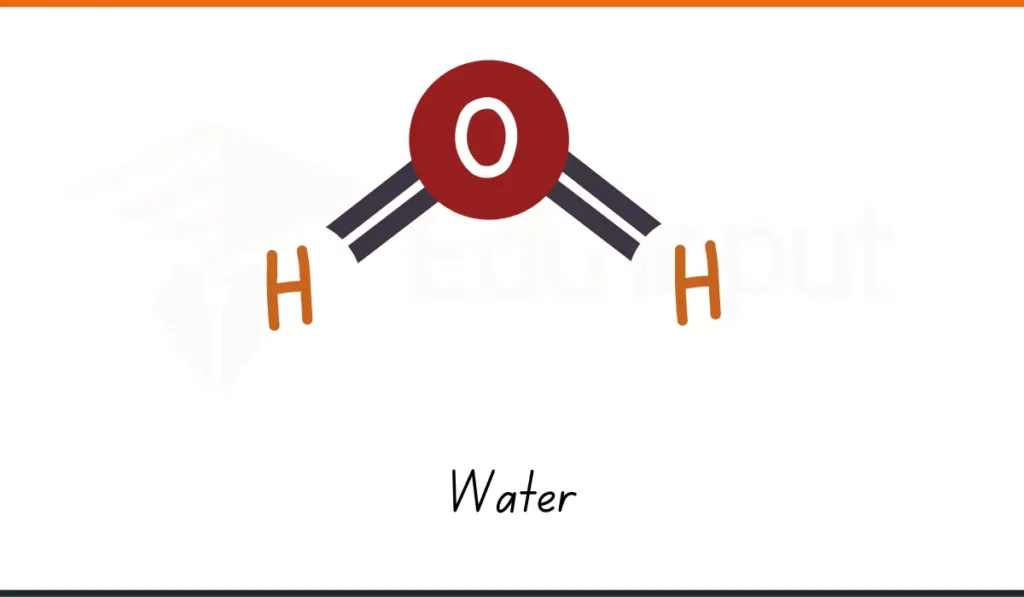
1. Water
- Formula: H₂O
- Composition: 2 hydrogen atoms, 1 oxygen atom
- Usage: Water (H₂O), a vital compound, is used for drinking, cooking, and cleaning at home.
- Why It Matters: Water is used every day. It is one of the simplest chemical compounds.
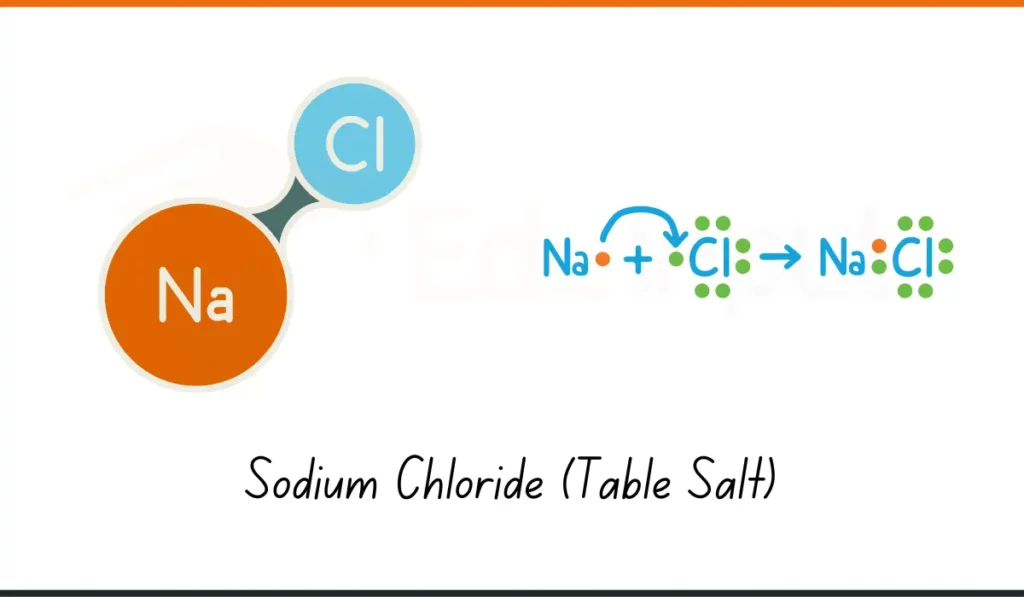
2. Sodium Chloride (Table Salt)
- Formula: NaCl
- Composition: 1 sodium atom, 1 chlorine atom
- Usage: Sodium chloride (NaCl) is used to season food and preserve it in homes.
- Why It Matters: It adds taste and keeps food fresh. A common chemical in daily life.
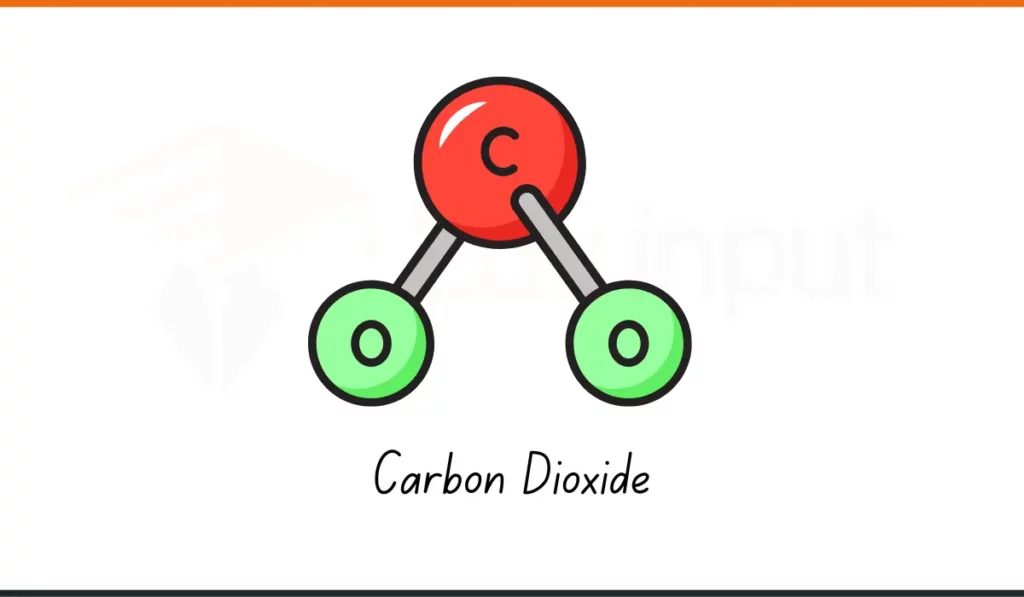
3. Carbon Dioxide
- Formula: CO₂
- Composition: 1 carbon atom, 2 oxygen atoms
- Usage: Carbon dioxide (CO₂) is used in fizzy drinks and in fire extinguishers.
- Why It Matters: It helps in safety and drinks. It is simple compound used every day.
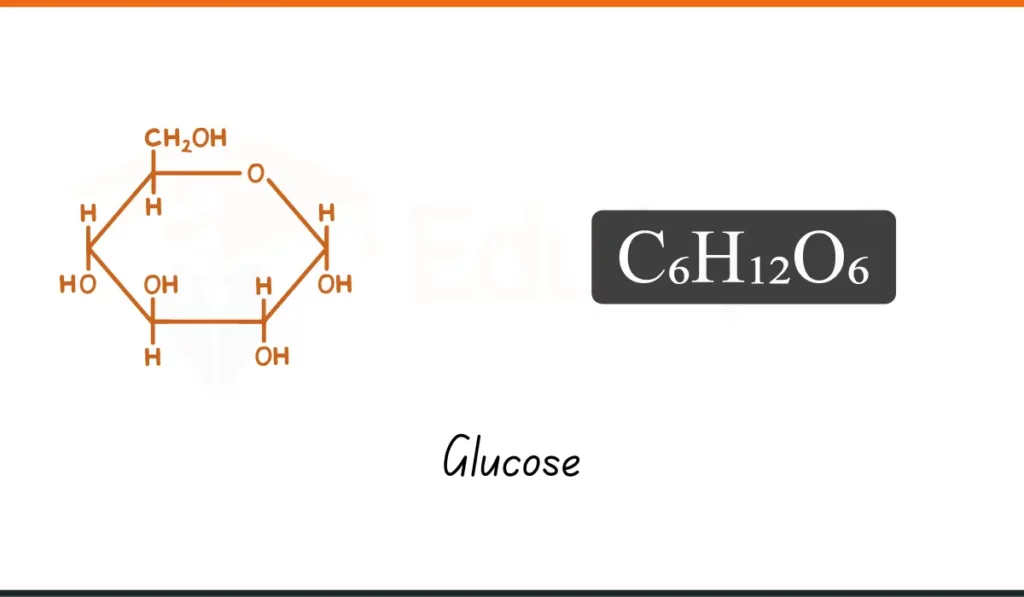
4. Glucose
- Formula: C₆H₁₂O₆
- Composition: 6 carbon, 12 hydrogen, 6 oxygen atoms
- Usage: Glucose (C₆H₁₂O₆) gives energy to our body and is found in food like fruits.
- Why It Matters: It powers our body. A basic chemical formula in daily life.
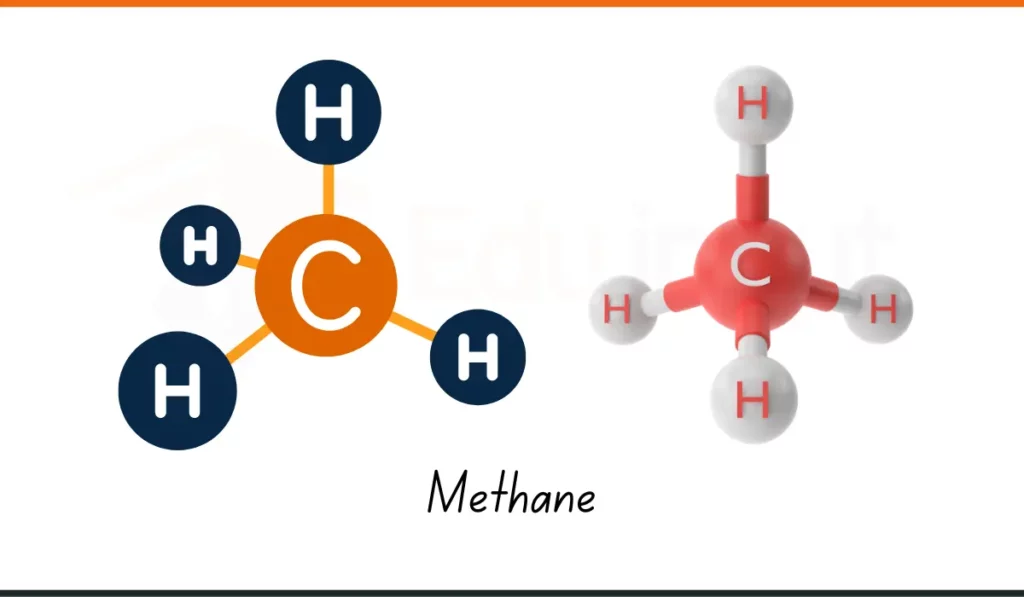
5. Methane
- Formula: CH₄
- Composition: 1 carbon atom, 4 hydrogen atoms
- Usage: Methane (CH₄) is used as a fuel for heating and cooking in many homes.
- Why It Matters: It gives energy for cooking. A useful example of chemical compounds at home.
6. Isopropyl Alcohol (C₃H₈O)
- Formula: C₃H₈O
- Composition: 3 carbon, 8 hydrogen, 1 oxygen atom
- Usage: Isopropyl alcohol (C₃H₈O) cleans wounds and kills germs on surfaces.
- Why It Matters: It keeps things germ-free. A key chemical compound for hygiene.
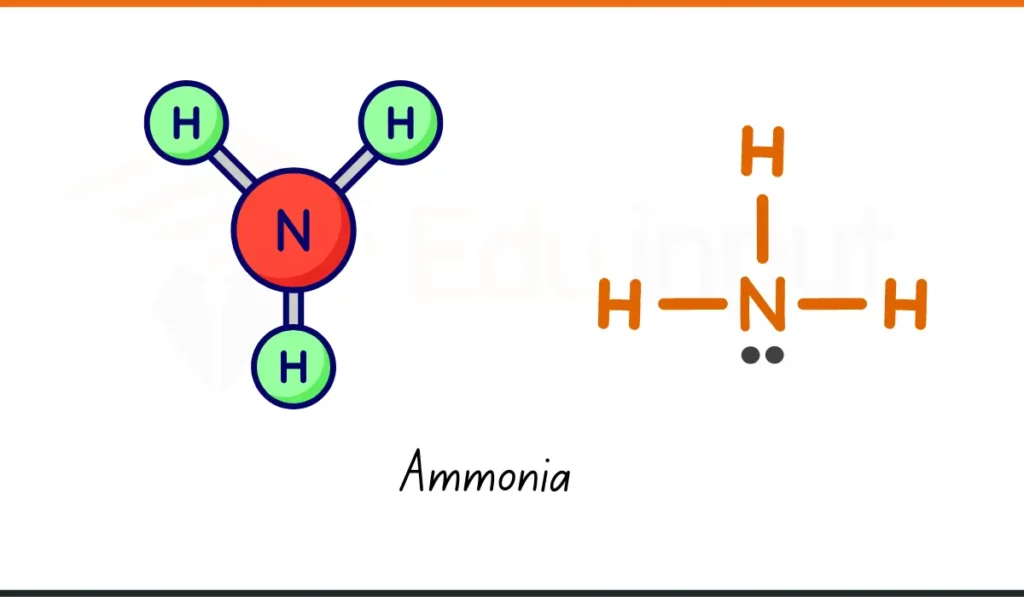
7. Ammonia
- Formula: NH₃
- Composition: 1 nitrogen, 3 hydrogen atoms
- Usage: Ammonia (NH₃) is used in cleaners and in making fertilizers for plants.
- Why It Matters: It helps grow food and clean. A useful chemical compound.
8. Calcium Chloride
- Formula: CaCl₂
- Use: It melts ice on roads and keeps dust down.
- Calcium chloride (CaCl₂) melts ice on roads, ensuring safe winter travel (compounds in industry).
- Why It Matters: It keeps roads safe. A cold-weather chemical compound.
9. Ethylene (Ethene)
Chemical Formula: C₂H₄
Composition: 2 carbon atoms, 4 hydrogen atoms
10. Calcium Carbonate
Chemical Formula: CaCO₃
Composition: 1 calcium atom, 1 carbon atom, 3 oxygen atoms
11. Hydrochloric Acid
Chemical Formula: HCl
Composition: 1 hydrogen atom, 1 chlorine atom
12. Nitric Acid
- Formula: HNO₃
- Use: It is used to make fertilizers.
- Nitric acid (HNO₃) is used in fertilizers to increase crop yields.
- Why It Matters: It helps crops grow. A farming chemical formula.
13. Sucrose (Table Sugar)
Chemical Formula: C₁₂H₂₂O₁₁
Composition: 12 carbon atoms, 22 hydrogen atoms, 11 oxygen atoms
14. Acetic Acid
- Formula: CH₃COOH
- Composition: 2 carbon, 4 hydrogen, 2 oxygen atoms
- Usage: Vinegar (CH₃COOH) is used for cleaning and flavoring food like salads.
- Why It Matters: It is used often in kitchens. A simple chemical in daily use..
15. Methanol
Chemical Formula: CH₃OH
Composition: 1 carbon atom, 4 hydrogen atoms, 1 oxygen atom
16. Glycerol
Chemical Formula: C₃H₈O₃
Composition: 3 carbon atoms, 8 hydrogen atoms, 3 oxygen atoms
17. Ammonium Nitrate
Chemical Formula: NH₄NO₃
Composition: 4 hydrogen atoms, 1 nitrogen atom, 3 oxygen atoms
18. Benzene
Chemical Formula: C₆H₆
Composition: 6 carbon atoms, 6 hydrogen atoms
19. Potassium Hydroxide
Chemical Formula: KOH
Composition: 1 potassium atom, 1 oxygen atom, 1 hydrogen atom
20. Sodium Bicarbonate (Baking Soda)
- Formula: NaHCO₃
- Composition: 1 sodium, 1 hydrogen, 1 carbon, 3 oxygen atoms
- Usage: Baking soda (NaHCO₃) helps bake fluffy bread and clean kitchen surfaces.
- Why It Matters: It helps in cooking and cleaning. A useful household chemical formula.
21. Hydrogen Peroxide
- Formula: H₂O₂
- Use: It cleans wounds and treats water.
- Hydrogen peroxide (H₂O₂) cleans wounds and purifies water, a versatile compound.
- Why It Matters: It kills germs and purifies. A common chemical formula in daily life.
22. Sodium Hydroxide (Caustic Soda)
- Formula: NaOH
- Composition: 1 sodium, 1 oxygen, 1 hydrogen atom
- Usage: Sodium hydroxide (NaOH) is used in making soap and cleaning drains.
- Why It Matters: It makes soap and cleans well. A strong chemical compound in industry.
23. Urea
- Formula: CH₄N₂O
- Use: Urea is used as plant food in farms.
- Urea (CH₄N₂O) enriches soil, feeding global crops.
- Why It Matters: It feeds plants. A common compound in agriculture.
24. Aspirin
Chemical Formula: C₉H₈O₄
Composition: 9 carbon atoms, 8 hydrogen atoms, 4 oxygen atoms
25. Caffeine
Chemical Formula: C₈H₁₀N₄O₂
Composition: 8 carbon atoms, 10 hydrogen atoms, 4 nitrogen atoms, 2 oxygen atoms
26. Carbon Tetrachloride
Chemical Formula: CCl₄
Composition: 1 carbon atom, 4 chlorine atoms
27. Sodium Carbonate (Soda Ash)
Chemical Formula: Na₂CO₃
Composition: 2 sodium atoms, 1 carbon atom, 3 oxygen atoms
28. Methane
Chemical Formula: CH₄
Composition: 1 carbon atom, 4 hydrogen atoms
29. Sulfur Hexafluoride
- Formula: SF₆
- Use: It is used in electric tools and air tests.
- Sulfur hexafluoride (SF₆) insulates power grids and tracks air currents.
- Why It Matters: It helps with electricity and weather. A special compound in science.
30. Lactic Acid
Chemical Formula: C₃H₆O₃
Composition: 3 carbon atoms, 6 hydrogen atoms, 3 oxygen atoms
What elements are compounds?
Compounds are substances formed when two or more elements chemically bond together. Here are a few examples of common elements that form compounds:
- Hydrogen (H) – Forms compounds like water (H₂O) and hydrogen chloride (HCl).
- Oxygen (O) – Found in compounds such as carbon dioxide (CO₂) and glucose (C₆H₁₂O₆).
- Carbon (C) – Forms a variety of compounds, including methane (CH₄) and ethylene (C₂H₄).
- Nitrogen (N) – Commonly found in compounds like ammonia (NH₃) and nitric acid (HNO₃).
- Sodium (Na) – Forms compounds such as sodium chloride (NaCl) and sodium bicarbonate (NaHCO₃).
- Chlorine (Cl) – Found in compounds like sodium chloride (NaCl) and chlorinated solvents (e.g., CCl₄).
- Calcium (Ca) – Forms compounds such as calcium carbonate (CaCO₃) and calcium sulfate (CaSO₄).
- Sulfur (S) – Commonly found in compounds like sulfur dioxide (SO₂) and hydrogen sulfide (H₂S).
These elements combine to create a wide range of chemical compounds.
what are 10 common chemicals used at home?
Here are 10 common chemicals used at home along with their formulas:
Water – H₂O
Sodium Chloride (Table Salt) – NaCl
Vinegar (Acetic Acid) – CH₃COOH
Baking Soda (Sodium Bicarbonate) – NaHCO₃
Ammonia – NH₃
Hydrogen Peroxide – H₂O₂
Rubbing Alcohol (Isopropyl Alcohol) – C₃H₈O
Bleach (Sodium Hypochlorite) – NaClO
Calcium Carbonate (Found in Antacids) – CaCO₃
Citric Acid – C₆H₈O₇




Leave a Reply