Concentration Gradient-Definition, Types & Examples
A concentration gradient refers to the difference in the concentration of a particular substance between two regions or locations during the process of diffusion. This gradient serves as the driving force behind the movement of molecules from areas of higher concentration to areas of lower concentration.
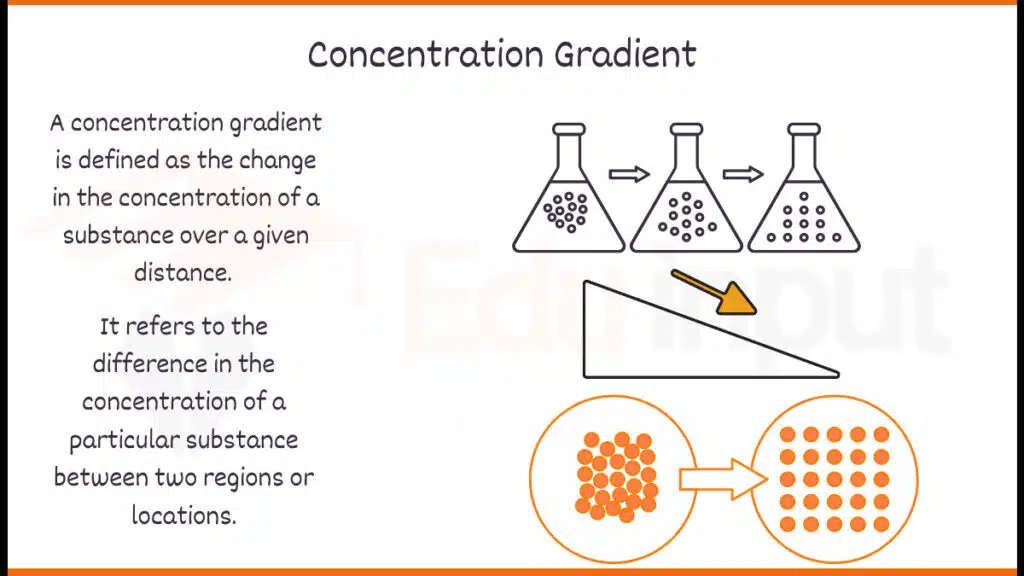
A concentration gradient is defined as the change in the concentration of a substance over a given distance. It is typically expressed as the change in concentration per unit distance. Mathematically, the concentration gradient can be represented as:
Concentration Gradient = ΔC/Δx
Where:
- ΔC represents the change in concentration
- Δx represents the change in distance
The steeper the concentration gradient, the greater the driving force for the movement of molecules down the gradient, resulting in a faster rate of diffusion.
Types of Concentration Gradients
Here are a few types of Concentration Gradients:
1. Simple Concentration Gradient
This type of gradient occurs when there is a difference in the concentration of a single substance between two regions within an organism or a cell. For example, the diffusion of a dye or a solute from a higher concentration region to a lower concentration region in a solution or the diffusion of oxygen from the lungs (higher concentration) into the bloodstream (lower concentration).
2. Compound Concentration Gradient
In this case, the gradient involves multiple substances with different concentrations in different regions. This type of gradient is commonly observed in biological systems, where various molecules and ions are present at varying concentrations across cell membranes or within tissues.
In biological systems, concentration gradients often involve multiple substances with different concentrations across cell membranes or within tissues.
For instance, the diffusion of various nutrients, waste products, and signaling molecules across cell membranes is driven by their respective compound concentration gradients.
3. Electrochemical Gradient
This gradient occurs when there is a difference in the concentration of charged particles (ions) across a membrane or barrier. This gradient is particularly important in biological systems, as it involves the movement of charged particles (ions) across membranes. The electrochemical gradient combines the concentration gradient of ions and the electrical potential gradient created by the difference in charge distribution across the membrane.
This gradient is important for processes such as nerve impulse conduction, muscle contraction, and the active transport of ions by proteins like the sodium-potassium pump. The electrochemical gradient combines the concentration gradient and the electrical potential gradient, which arises from the difference in charge distribution across the membrane.
4. Osmotic Gradient
Osmosis is a special case of diffusion, where the concentration gradient involves water molecules moving across a semi-permeable membrane. Water moves from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). This gradient is essential for maintaining the proper water balance and volume regulation in cells and tissues.
Examples of Concentration Gradients
Here are a few examples of how Concentration Gradients helps in different processes:
- Nutrient Uptake – In biological systems, concentration gradients play role in the uptake of essential nutrients by cells. For instance, glucose diffuses from the bloodstream (higher concentration) into the cells (lower concentration) to provide energy for cellular processes.
- Gas Exchange – The diffusion of gases, such as oxygen and carbon dioxide, across biological membranes is driven by concentration gradients. In the lungs, oxygen diffuses from the air (higher concentration) into the bloodstream (lower concentration), while carbon dioxide diffuses in the opposite direction.
- Osmosis – Osmosis is a special case of diffusion, where the concentration gradient involves water molecules. Water moves across a semi-permeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration).
- Ion Transport – Concentration gradients of ions, such as sodium and potassium, are maintained across cell membranes by active transport mechanisms like the sodium-potassium pump. These gradients are essential for various cellular processes, including nerve impulse conduction and muscle contraction.
- Dialysis – In medical applications, dialysis machines utilize concentration gradients to remove waste products and excess water from the blood of individuals with kidney failure. The concentration gradient between the blood and the dialysis solution drives the diffusion of these unwanted substances across a semi-permeable membrane.






Leave a Reply